A few years ago, I wrote that we do not know how to draw ferrocene or a nitro group. (Still true.) Is the situation with polycyclic aromatic hydrocarbons any better?
Take hexabenzo[bc,ef,hi,kl,no,qr]coronene, one of the subjects of the single-molecule visualisation study published last week in Science [1]. One way to draw it shown in diagram (a):
 |
| (a) |
I chose this one (out of many other possible Kekulé representations) because I can reproduce it on a paper napkin (beermat, Post-it note, you name it). If you look carefully, you will notice that the central ring and the six outermost rings are connected with single bonds.
 |
| (b) |
Continuing the paper-napkin-doodle argument, it is even easier to draw a circle inside of each ring as in all-delocalised representation (b). However, that would not be a preferred diagram from IUPAC point of view [2, GR-6.5]: for example, benzene ⏣ is acceptable but ⌬ is preferred. Moreover, “it is generally not acceptable to use curves in two adjacent fused rings”. Still, I’d stick with circles.
The question is, do I have to draw a circle within each ring? Of course not. If I draw seven aromatic rings and connect the with single bonds as shown in (c), the resulting structure will be the same. In this way, I can even save some ink (graphite, chalk, etc.)
 |
| (c) |
Without the circles, the six rings that surround the central ring in (c) start to look, well, more empty. Using the noncontact atomic force microscopy (NC-AFM), the team behind the study [1] were able to show (and in this case “to show” really means “to show”), that those rings are indeed slightly larger. The C—C bonds in the central ring (i-bonds, 1.417 Å) are 0.03 Å shorter than the bonds connecting that ring with the six outermost rings (j-bonds, 1.447 Å).
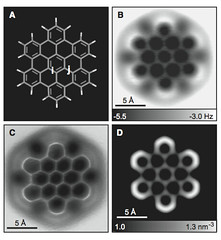
- Gross, L., Mohn, F., Moll, N., Schuler, B., Criado, A., Guitián, E., Peña, D., Gourdon, A. and Meyer, G. (2012) Bond-order discrimination by atomic force microscopy. Science 337, 1326—1329.
- Brecher, J. (2008) Graphical representation standards for chemical structure diagrams (IUPAC Recommendations 2008). Pure Appl. Chem. 80, 277—410.