After hours spent looking in my books and searching the internet, I came to the conclusion that chemists talk about chains and rings without explaining what they mean. The only definition I found so far, viz. that of Gold Book, is specific for polymers and seems to be too complex to be used in general chemical nomenclature:
The whole or part of a macromolecule, an oligomer molecule or a block, comprising a linear or branched sequence of constitutional units between two boundary constitutional units, each of which may be either an end-group, a branch point or an otherwise-designated characteristic feature of the macromolecule. |
(1) |
On the other hand, general dictionary definitions of (chemical) chains are not precise enough. For example, Collins English Dictionary defines chain (chemistry) as
two or more atoms or groups bonded together so that the configuration of the resulting molecule, ion, or radical resembles a chain. |
(2) |
whereas Merriam-Webster says that it is
a number of atoms or chemical groups united like links in a chain. |
(3) |
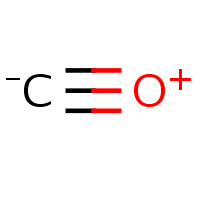
So chain (chemistry) is like a chain. Is it?














![[Cr(O)2(O-)2]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEiMl79HNr0UpiyYYGh19PXfrVS8tgjZpGFAXZVCc4UOf9mkbRi22X04ihTqlc4kIt3MhjkoadFSu1o4S_bm2B_MrMmvKwlJB__guvw6LTHk2KhQpN1daOMI2lDyeUkHefZXhMy2pw/s320/%5BCr(O)2(O-)2%5D.png)
![[Cr(O)4]2-](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEig_6nDbBjkdqedyqMZqcaqsMjAKQTNTh6qfYC695EGI2cTTKtyAbJh13mjeVrv_7wLrr6mcW1FMJVxrR1n9JCZHH9R5Dwb-gWMGpJSDl2_VwEirZWvOGQv359HmZuN3mLmDDS19A/s320/%5BCr(O)4%5D2-.png)
![[Cr(2+)(O-)4]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhcX_-K6YsWcufdFVEykYISID9dKdDFxNYAtXgcZvGq4cOeyXrwgE4DhOAAQVRu23tiP0soKyY7s05pGIabeMwIZX1YoPhL73RGw6zuauvpkJ9w5DQMnLqzS9PTzcO0tsKc1yoYVg/s320/%5BCr(2+)(O-)4%5D.png)

6_charges.png)
6.png)
O.png)
2.png)






![3-[hydroxy(oxido)phosphoranyl]pyruvic acid](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEj4EOt2L_5jcMhNbgn4JaWfKl-49pWwfiawIMueHGFURAD7kBFWozYQr-DrmneMCv2tF-8lST5RFOQZg6w5ncJbfjh5iXS87ZKqo5idU87wsDVn24gxhhyphenhyphentHKwP6d4EmyJPAlXF7A/s320/CHEBI18007.png)












