The first step of biological methane formation from carbon dioxide is the reduction of CO2 to form N-formylmethanofuran from methanofuran. This reaction is catalysed by formylmethanofuran dehydrogenase (EC 1.2.99.5). There are two types of this enzyme in methanogenic archaea, a tungsten iron—sulphur protein (Fwd) and a molybdenum iron—sulphur protein (Fmd).
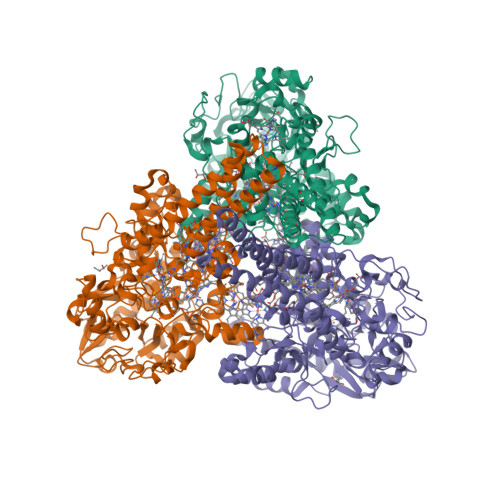
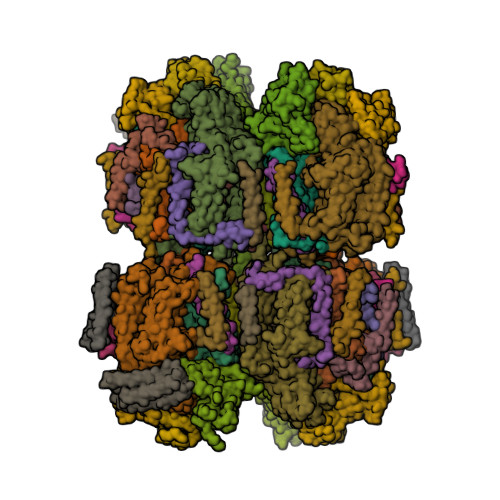
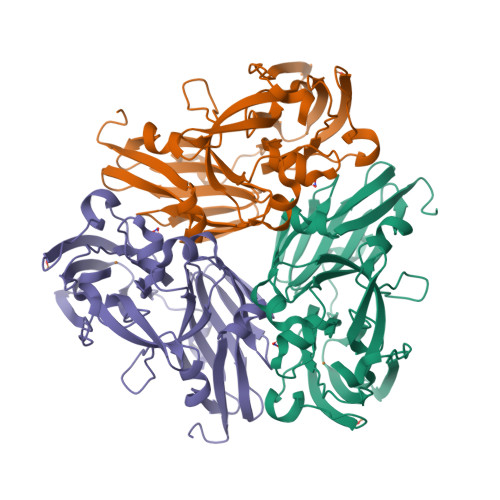
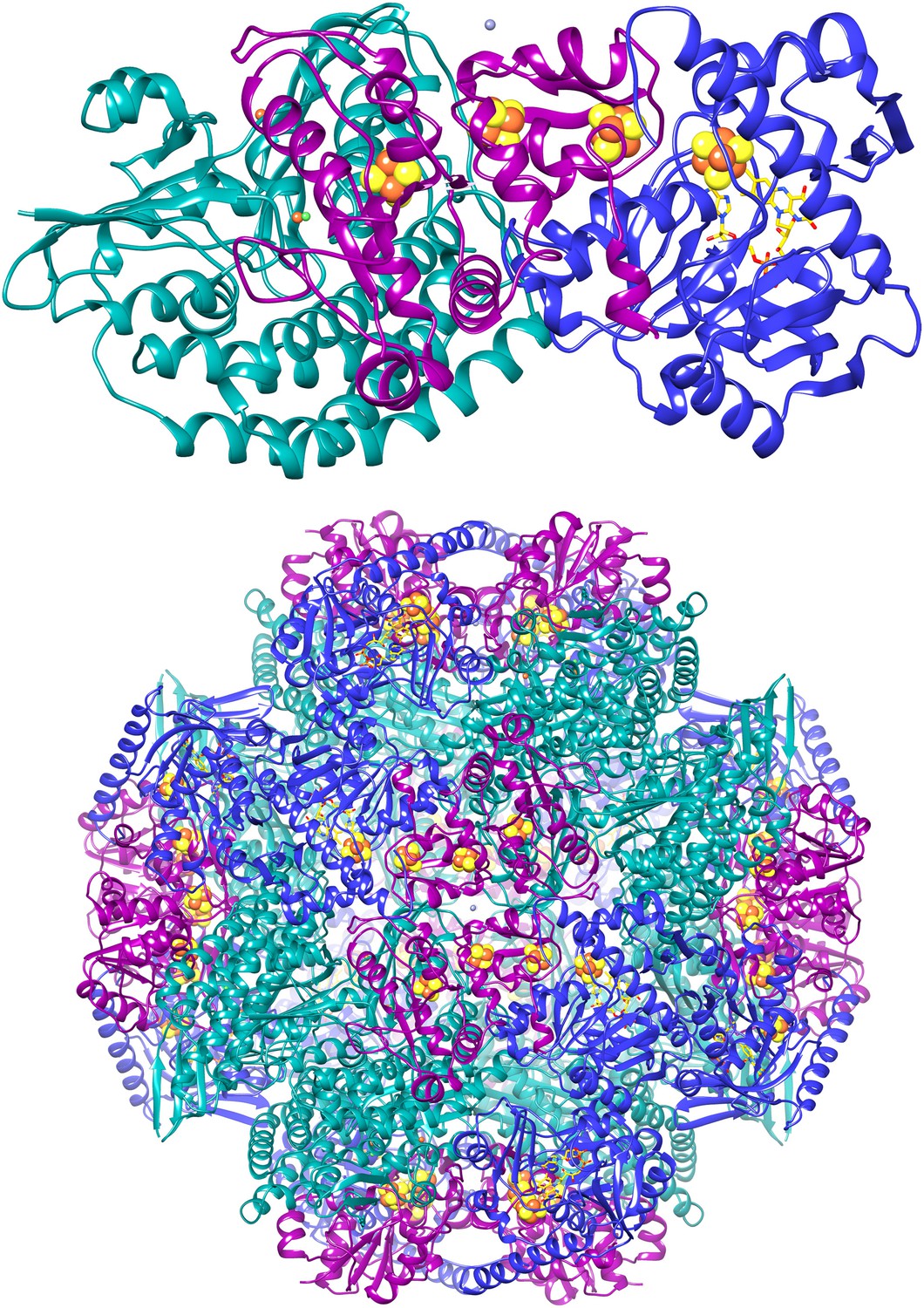
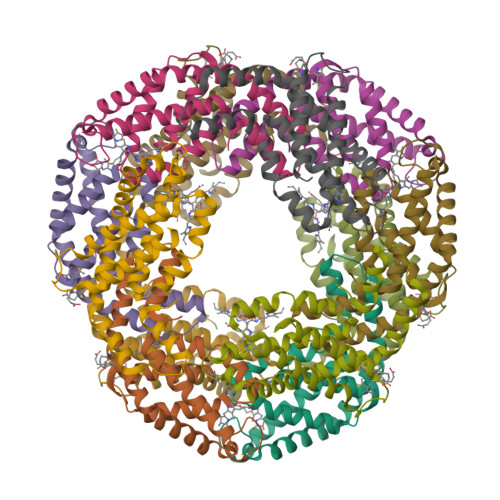
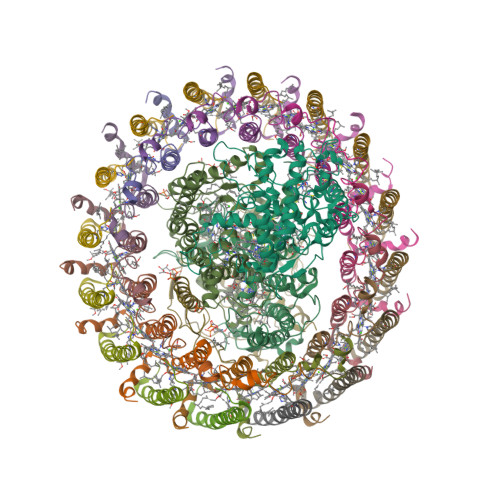
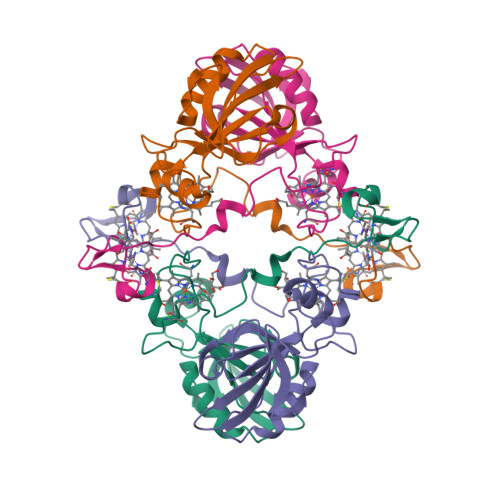
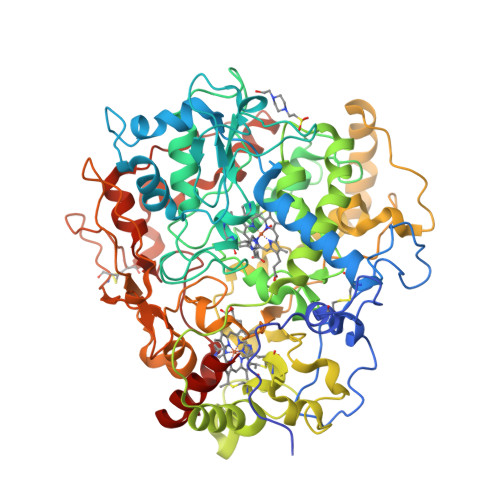
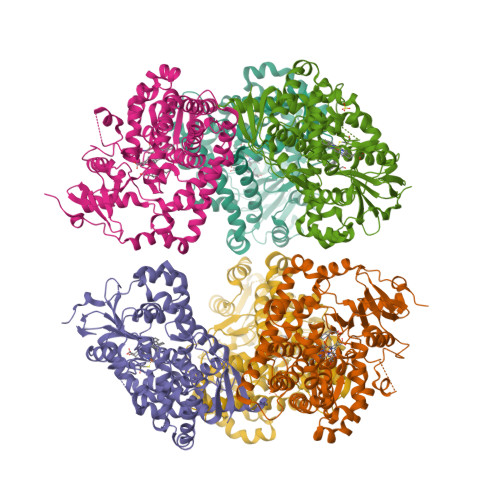
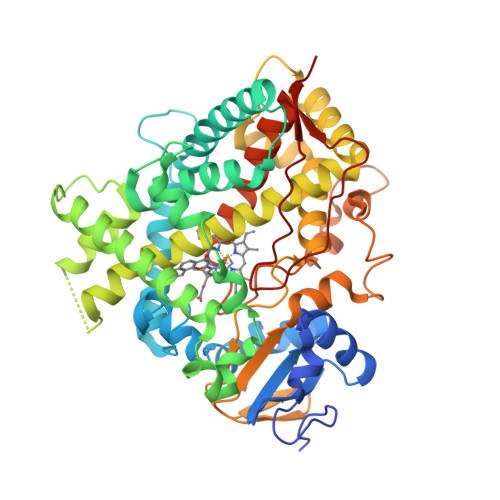
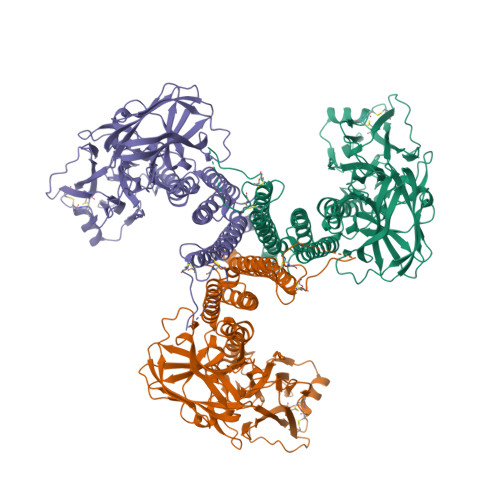
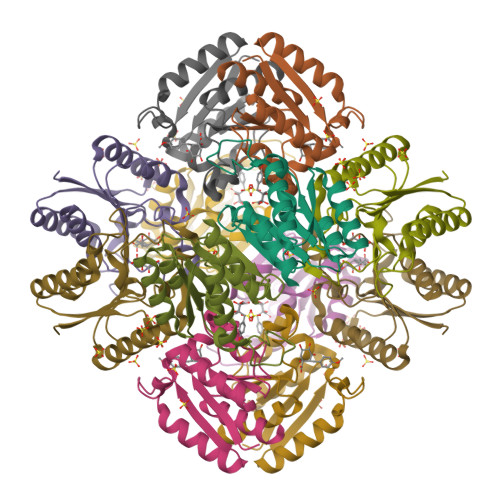
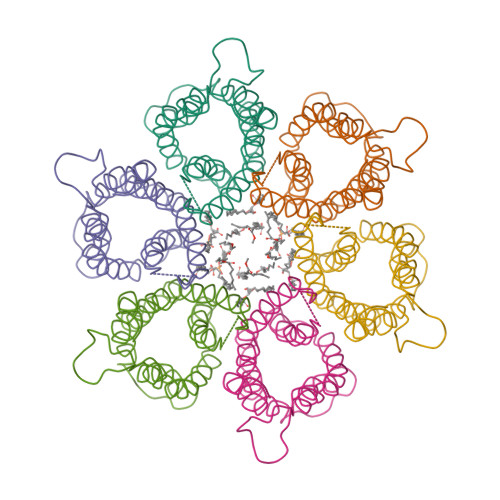
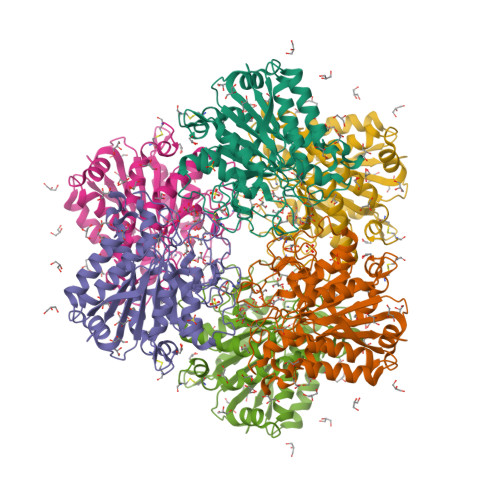
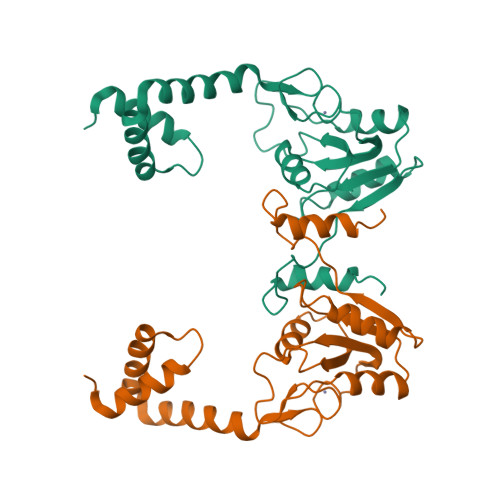
Wagner et al. [1] determined the X-ray structures of a Fwd enzyme from the thermophilic methanogenic archaeon Methanothermobacter wolfeii in several crystal forms [2—4]. To any bioinorganic chemist this metalloprotein should look like a treasure trove: every FwdABCDFG heterohexamer has got a mononuclear tungsten centre, a dinuclear zinc centre, and quite a few iron—sulphur clusters. The enzyme exists as either a dimer or a tetramer of the FwdABCDFG heterohexamers. The authors suggest that the 24-meric complex (FwdABCDFG)4 is a physiologically active form. It contains 46 (yes, forty-six) [Fe4S4] clusters which form an electron wire between the redox-active tungsten centres. The function of this wire remains unclear though.


- Wagner, T., Ermler, U. and Shima, S. (2016) The methanogenic CO2 reducing-and-fixing enzyme is bifunctional and contains 46 [4Fe-4S] clusters. Science 354, 114—117.
- PDB:5T5I
- PDB:5T5M
- PDB:5T61