Some years ago, I’ve circulated this list among my colleagues at the EBI. I think it may be of interest to the readers of this blog as well. Here goes:
Copper was named after Cyprus, francium and gallium after France, germanium after Germany, polonium after Poland, ruthenium after Russia, and americium after (the United States of) America. Magnesium was named after Magnesia region in Greece, hassium after the land of Hesse (Hessen) in Germany, and californium after California. In addition, europium got his name after (continent of) Europe while the names of both scandium and thulium have something to do with Scandinavia. However, indium was named not after India but because of blue (indigo) line in its atomic spectrum. As for cities and villages, lutetium was named after Paris, hafnium after Copenhagen, holmium after Stockholm, strontium after Strontian in Scotland, berkelium after Berkeley in California, dubnium after Dubna in Russia*, and rather unpronounceable darmstadtium after Darmstadt in Germany. Four elements (yttrium, erbium, terbium, ytterbium) took their names after otherwise little known Ytterby in Sweden. Rhenium was named after the (river) Rhine. All the place-name elements, except for germanium, are metals.
Apart from Argentina, I cannot think of any other country named after a metal or any other element (unless you count Cyprus again, which well could have been named after copper; the history is not very clear here). According to the Wikipedia, the smallest of Canary Islands, El Hierro (Spanish for ‘iron’) originally had a name ‘Hero’, later mutated into ‘Hierro’ and further latinised as ‘Ferro’ while having nothing to do with iron. Lead, South Dakota also has nothing to do with lead (metal). I am sure there are plenty of placenames featuring coinage metals (gold, silver, copper) in a variety of languages, but I better stop for now.
* Dubna is the only town I know that has both
flag and
coat of arms featuring a ‘popular culture’ atom symbol
⚛


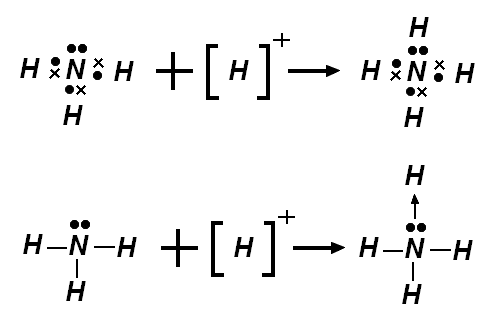


















![[Cr(O)2(O-)2]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEiMl79HNr0UpiyYYGh19PXfrVS8tgjZpGFAXZVCc4UOf9mkbRi22X04ihTqlc4kIt3MhjkoadFSu1o4S_bm2B_MrMmvKwlJB__guvw6LTHk2KhQpN1daOMI2lDyeUkHefZXhMy2pw/s320/%5BCr(O)2(O-)2%5D.png)
![[Cr(O)4]2-](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEig_6nDbBjkdqedyqMZqcaqsMjAKQTNTh6qfYC695EGI2cTTKtyAbJh13mjeVrv_7wLrr6mcW1FMJVxrR1n9JCZHH9R5Dwb-gWMGpJSDl2_VwEirZWvOGQv359HmZuN3mLmDDS19A/s320/%5BCr(O)4%5D2-.png)
![[Cr(2+)(O-)4]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhcX_-K6YsWcufdFVEykYISID9dKdDFxNYAtXgcZvGq4cOeyXrwgE4DhOAAQVRu23tiP0soKyY7s05pGIabeMwIZX1YoPhL73RGw6zuauvpkJ9w5DQMnLqzS9PTzcO0tsKc1yoYVg/s320/%5BCr(2+)(O-)4%5D.png)


6_charges.png)
6.png)



O.png)
2.png)