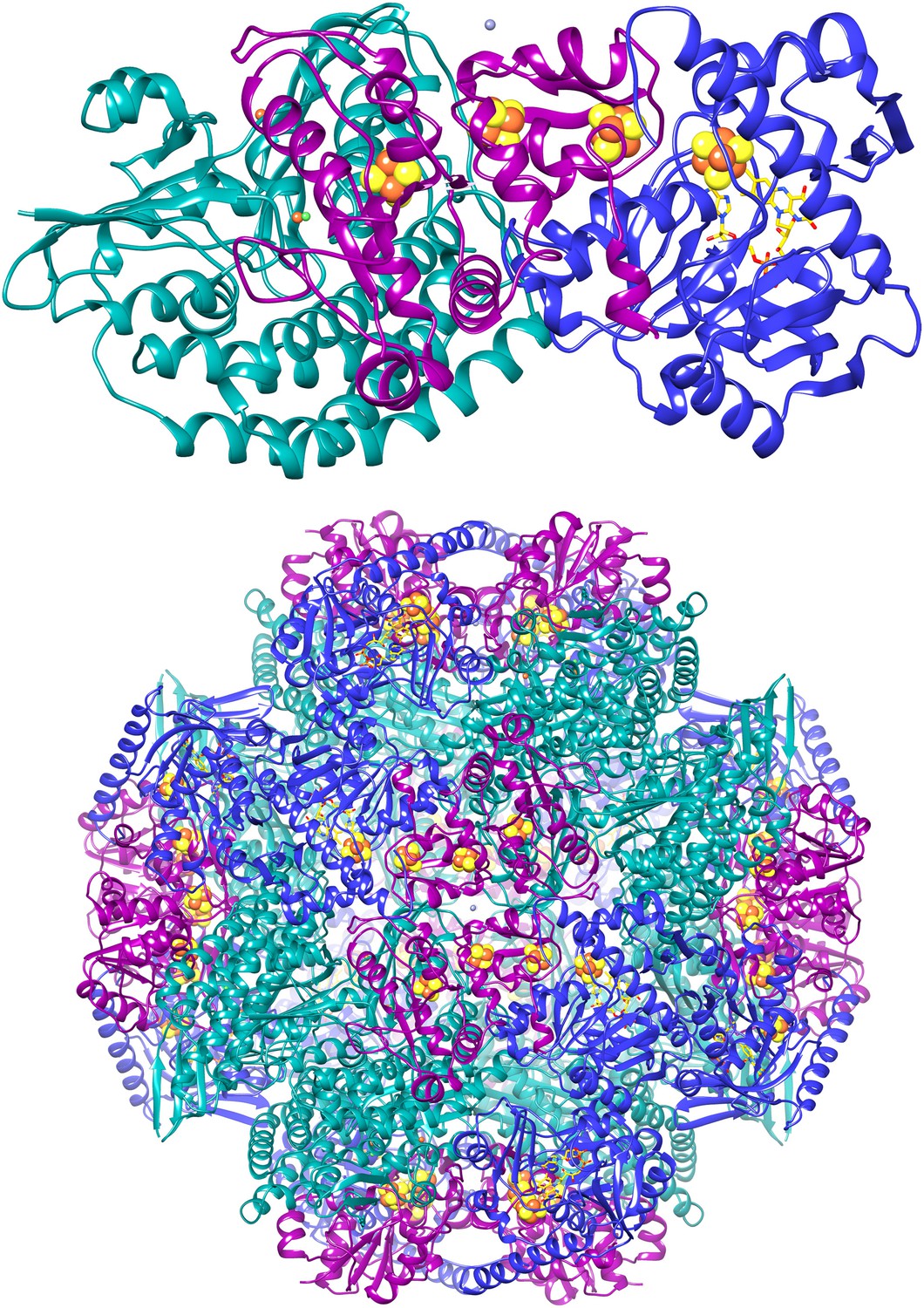
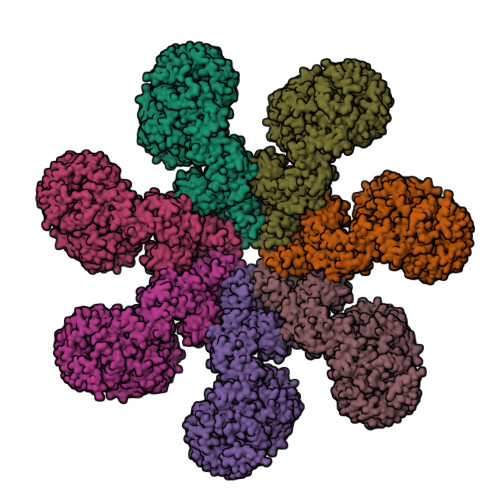
The apoptotic protease-activating factor 1 (Apaf-1) exists in normal cells as an autoinhibited monomer. Upon binding to cytochrome c and dATP, Apaf-1 forms a heptameric complex known as the apoptosome. Zhou et al. report an atomic structure of an intact human Apaf-1 apoptosome at 3.8 Å resolution determined by single-particle, cryo-electron microscopy [1, 2].
- Zhou, M., Li, Y., Hu, Q., Bai, X.-c., Huang, W., Yan, C., Scheres, S.H.W. and Shi, Y. (2015) Atomic structure of the apoptosome: mechanism of cytochrome c- and dATP-mediated activation of Apaf-1. Genes & Development 29, 2349—2361.
- PDB:3JBT