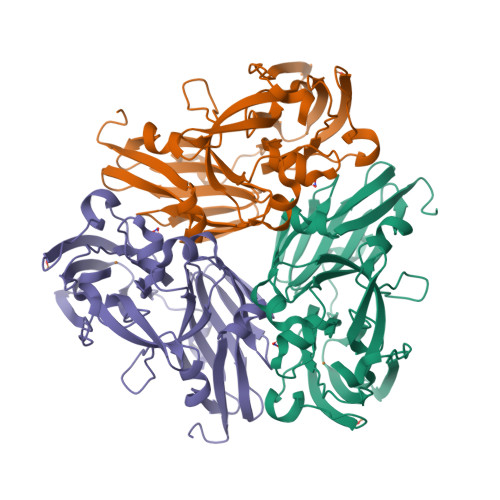
Fukuda and Inoue [1] have determined the crystal structure of the C135A mutant of thermostable copper nitrite reductase (CuNIR) from Geobacillus thermodenitrificans in complex with nitrite to 1.55 Å resolution. Interestingly, this high-temperature (320 K) structure [2] displays a near-bidentate binding mode of nitrite distinct from a monodentate mode in a cryogenic structure [3]:
To our knowledge, this is the first case in which the difference in substrate binding modes between cryogenic and high-temperature structures has been visualized by crystallography.
The copper site geometries are given in Table 1.
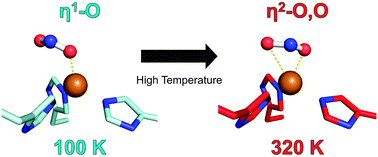
Table 1 (adapted from [4])
| Cu—Ligand Distances (Å) | 3X1N (320 K) | 3WKP (100 K) |
|---|---|---|
| I. Type 1 Cu—residue distances | ||
| T1Cu—H95Nδ1 | 2.08 | 2.14 |
| T1Cu—H143Nδ1 | 2.01 | 1.96 |
| T1Cu—M148Sδ | 2.13 | 2.07 |
| II. Type 2 Cu—residue distances | ||
| T2Cu—H100Nε2 | 2.07 | 1.96 |
| T2Cu—H134Nε2 | 2.00 | 1.95 |
| T2Cu—H294Nε2 | 1.98 | 2.01 |
| T2Cu—water | 2.02 | n/a |
| III. Type 2 Cu—nitrite distances | ||
| T2Cu—Oproximal | 2.13 | 1.97 |
| T2Cu—N | 2.21 | 2.85 |
| T2Cu—Odistal | 2.52 | 3.41 |
- Fukuda, Y. and Inoue, T. (2015) High-temperature and high-resolution crystallography of thermostable copper nitrite reductase. Chemical Communications 51, 6532—6535.
- PDB:3X1N
- PDB:3WKP
- Fukuda, Y. and Inoue, T. (2015) High-temperature and high-resolution crystallography of thermostable copper nitrite reductase. Electronic Supplementary Material.