Five years ago, Schemberg et al. reported the crystal structure of molybdenum/tungsten storage protein from Azotobacter vinelandii complexed with polyoxotungstates [1, 2].
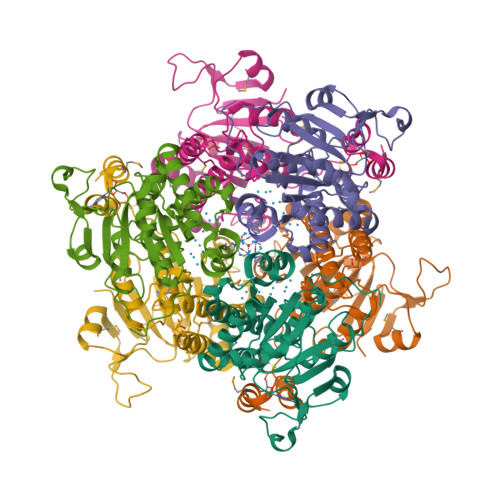
Now Kowalewski et al. report the 1.6 Å X-ray structure of the same protein containing a variety of polyoxomolybdate clusters, from Mo3 to Mo8 [3].
Some N2-fixing bacteria prolong the functionality of nitrogenase in molybdenum starvation by a special Mo storage protein (MoSto) that can store more than 100 Mo atoms. The presented 1.6 Å X-ray structure of MoSto from Azotobacter vinelandii reveals various discrete polyoxomolybdate clusters, three covalently and three noncovalently bound Mo8, three Mo5–7, and one Mo3 clusters, and several low occupied, so far undefinable clusters, which are embedded in specific pockets inside a locked cage-shaped (αβ)3 protein complex. <...> The formed polyoxomolybdate clusters of MoSto, not detectable in bulk solvent, are the result of an interplay between self- and protein-driven assembly processes that unite inorganic supramolecular and protein chemistry in a host–guest system.

- Schemberg, J., Schneider, K., Demmer, U., Warkentin, E., Müller, A. and Ermler, U. (2007) Towards biological supramolecular chemistry: a variety of pocket-templated, individual metal oxide cluster nucleations in the cavity of a Mo/W-storage protein. Angewandte Chemie International Edition 46, 2408—2413.
- PDB:2OGX
- Kowalewski, B., Poppe, J., Demmer, U., Warkentin, E., Dierks, T., Ermler, U. and Schneider, K. (2012) Nature’s polyoxometalate chemistry: X-ray structure of the Mo storage protein loaded with discrete polynuclear Mo–O clusters. J. Am. Chem. Soc. 134, 9768—9774.




