Quadruple and higher order metal—metal bonds are known for transition metals, lanthanoids and actinoids. But for main group elements? Using four different computational methods, Shaik et al. [1] show that
C2 and its isoelectronic molecules CN+, BN and CB− (each having eight valence electrons) are bound by a quadruple bond. The bonding comprises not only one σ- and two π-bonds, but also one weak ‘inverted’ bond, which can be characterized by the interaction of electrons in two outwardly pointing sp hybrid orbitals.According to Shaik, the existence of the fourth bond in C2 suggests that it is not really diradical C22• [2]:
If C2 were a diradical it would immediately form higher clusters. I think the fact that you can isolate C2 tells you it has a barrier, small as it may be, to prevent that.
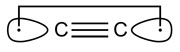
- Shaik, S., Danovich, D., Wu, W., Su, P., Rzepa, H.S. and Hiberty, P.C. Quadruple bonding in C2 and analogous eight-valence electron species. Nature Chemistry 4, 195—200.
- Extance, A. Calculations reveal carbon-carbon quadruple bond. Chemistry World, 29 January 2012.






