Remember the nitro group? Let us consider even ‘simpler’ case of carbon monoxide. Almost invariably, the chemical databases represent CO as a charge-separated molecule (a) even though it would be as correct to draw it with triple bond and two lone pairs (b). I guess the reason to chose the representation (a) is that the software used for drawing/validating concerns itself with electron accountancy of separate atoms rather than whole molecule.
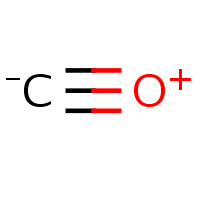 |  |
| (a) | (b) |
|---|
What about metal carbonyls? For instance, hexacarbonylvanadium, drawn with charge separation (c), looks really ugly. On the other hand, the software (e.g. ChemSketch) objects to the representation (d) (which, apparently, is preferred; cf. tetracarbonylnickel on p. 408 of IUPAC Recommendations) because it ‘wants’ the positive charge on triple-bonded oxygen.
6_charges.png) | 6.png) |
| (c) | (d) |
|---|







No comments:
Post a Comment