The Gold Book and the Silver Book (currently under revision) are two of the so-called colour books. Which kind of implies that gold and silver are colours. Not that IUPAC ran out of ‘real’ colours — e.g. they still could have chosen pink or yellow or black. For long time I thought that the reason behind naming the Gold Book ‘Gold Book’ was its (intended) role as a gold standard for chemical terminology. I was wrong. It was named in honour of Victor Gold (1922—1985), the British chemist who was the first author and compiler of the book. No such story with the Silver Book, I am afraid.

One of problems with the Gold Book (as opposed to other colour books) is that it deals with ‘general’ chemical nomenclature. Therefore, when it comes to terms which are different meanings in different fields of chemistry, the Gold Book gives more than one definition. Which one should be used? Take ligands. The definition 1 (coordination chemistry) is short and nice, while the definition 2 (biochemistry) is long and horrible. At least it mentions that in bioinorganic chemistry, one should be careful which definition to use.
In case of sulfides, there are three different meaning. Sulfides 1 (organic chemistry) is the replacement term for obsolete but less ambiguous ‘thioethers’, while sulfides 2 (inorganic chemistry) are “salts or other derivatives of hydrogen sulfide”. I am happy with salts but not with the “other derivatives”. Is sulfenic acid (IUPAC name ‘sulfanol’) a sulfide? As for sulfides 3, the definition goes “a term used in additive nomenclature”. Excellent.
Similarly, there is a consistency problem with related terms that are derived from different IUPAC recommendations. The entry for dipolar bond (1994) says:
The term is preferred to the obsolescent synonyms ‘coordinate link’, ‘coordinate covalence’, ‘dative bond’, ‘semipolar bond’.
And yet the more recent entry for dative bond (1999) does not mention that the term is obsolete. It is even states that
In spite of the analogy of dative bonds with covalent bonds, in that both types imply sharing a common electron pair between two vicinal atoms, the former are distinguished by their significant polarity, lesser strength, and greater length.
A textbook example of dative bond is the one in ammonium. Of course, all the N—H bonds are exactly the same, even if you choose to represent one of them with an arrow.
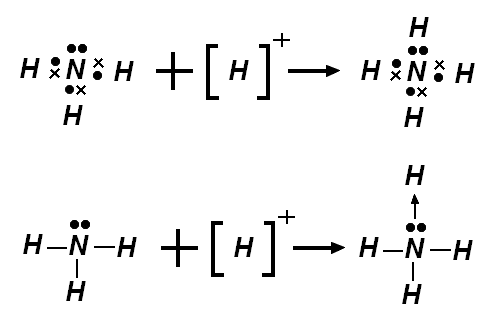







No comments:
Post a Comment