The crystal structures of rabbit P450 2B4 covalently bound to the mechanism-based inactivator 4-tert-butylphenylacetylene in closed (a) and open (b) conformations have been solved [1].
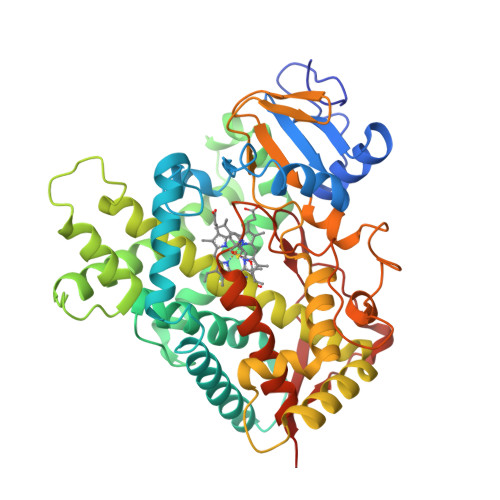 (a) | 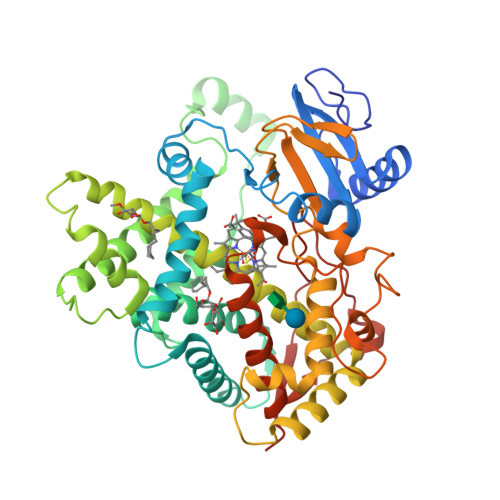 (b) |

- Gay, S.C., Zhang, H., Wilderman, P.R., Roberts, A.G., Liu, T., Li, S., Lin, H.-l., Zhang, Q., Woods, V.L., Jr., Stout, C.D., Hollenberg, P.F. and Halpert, J.R. (2011) Structural analysis of mammalian cytochrome P450 2B4 covalently bound to the mechanism-based inactivator tert-butylphenylacetylene: insight into partial enzymatic activity. Biochemistry 50, 4903—4911.







No comments:
Post a Comment