The X-ray crystal structure of Pseudomonas aeruginosa bacterioferritin (Pa-BfrB) in complex with bacterioferritin-associated ferredoxin (Pa-Bfd) has been solved at 2.0 Å resolution [1, 2].
As the first example of a ferritin-like molecule in complex with a cognate partner, the structure provides unprecedented insight into the complementary interface that enables the [2Fe-2S] cluster of Pa-Bfd to promote heme-mediated electron transfer through the BfrB protein dielectric (~18 Å), a process that is necessary to reduce the core ferric mineral and facilitate mobilization of Fe2+. The Pa-BfrB—Bfd complex also revealed the first structure of a Bfd, thus providing a first view to what appears to be a versatile metal binding domain ubiquitous to the large Fer2_BFD family of proteins and enzymes with diverse functions.
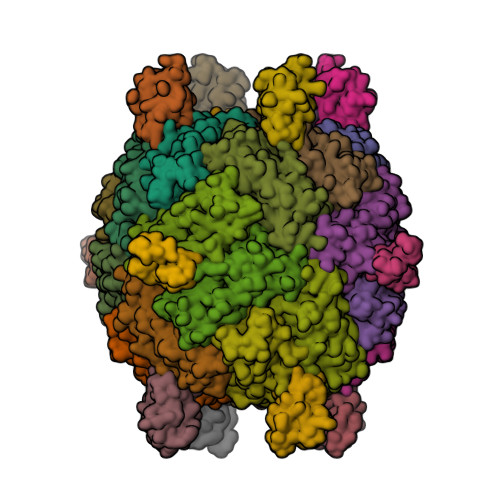
- Yao, H., Wang, Y., Lovell, S., Kumar, R., Ruvinsky, A.M., Battaile, K.P., Vakser, I.A. and Rivera, M. (2012) The structure of the BfrB—Bfd complex reveals protein—protein interactions enabling iron release from bacterioferritin. J. Am. Chem. Soc. 134, 13470—13481.
- PDB:4E6K







No comments:
Post a Comment