Magnetotactic bacteria (MTB) are a diverse group of prokaryotes that have a singular ability to align with geomagnetic field lines. This ability is due to special organelles called magnetosomes. Magnetosomes are composed of single-magnetic-domain nanocrystals of magnetite [Fe(II)Fe(III)2O4] or greigite [Fe(II)Fe(III)2S4] embedded in biological membrane.
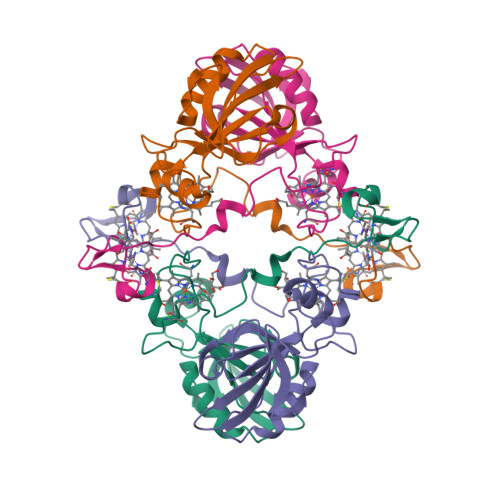
“Magnetochrome” is a name proposed in 2012 by Marina Siponen and co-authors for a cytochrome domain conserved within all known MTB and not found in any other species to date [1]. Recently, the crystal structure of the magnetosome-associated protein MamP has been solved at 1.8 Å resolution [2—4]. The minimal unit of MamP is a dimer. Each monomer consists of a PDZ domain fused to two magnetochrome domains. It was also shown in an in vitro mineralisation experiment that MamP functions as an iron oxidase mediating the iron(III) ferrihydrite production from iron(II) [2]:
- Siponen, M.I., Adryanczyk, G., Ginet, N., Arnoux, P. and Pignol, D. (2012) Magnetochrome: a c-type cytochrome domain specific to magnetotatic bacteria. Biochemical Society Transactions 40, 1319—1323.
- Siponen, M.I., Legrand, P., Widdrat, M., Jones, S.R., Zhang, W.-J., Chang, M.C.Y., Faivre, D., Arnoux, P. and Pignol, D. (2013) Structural insight into magnetochrome-mediated magnetite biomineralization. Nature 502, 681—684.
- PDB:4JJ0
- PDB:4JJ3







No comments:
Post a Comment