Let’s try to name the structure (a).
 |
| (a) |
|---|
|
We can give it a binary name carbon tetrabromide. We can name it additively tetrabromidocarbon.
Or we can call it in a completely different fashion: tetrabromomethane.
The latter name is constructed according to substitutive nomenclature. It is used mostly in the field of organic chemistry and allows for generating the derived names from a restricted set of parent names, or names of parent structures. The parent name becomes a root of a derived name, and the names of substituents become prefixes or suffixes. In organic chemistry, the term characteristic group, or functional group, is used for substituents that contain at least one heteroatom (that is, any atom other than carbon and hydrogen) and at most one carbon atom.
The most important type of parent structure is parent hydride. For example, methane (b) is said to be a parent hydride of tetrabromomethane (a). Methane derivatives can have one, two, three or all four hydrogen atoms substituted.
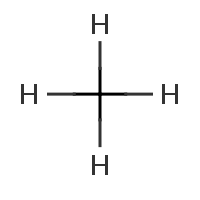 |
| (b) |
|---|
|
I must stress that the substitution of hydrogen is a purely formal exercise we undertake to generate a substitutive name. It has nothing to do with any substitution reaction. Likewise, we really only need parent hydride to derive substitutive names from it and we don’t even care if it does not exist in nature, as is the case with thallane (TlH3).
 |
 |
 |
| (c) | (d) | (e) |
|---|
|
One advantage of substitutive names over additive ones can be seen when we try to name structures (c) through (e): as we don’t have to list all the hydrogens explicitly, even in relatively simple cases of mononuclear hydride derivatives, the names can be significantly shorter.
But if that was all, I wouldn’t even bother with substitutive nomenclature. Its true power is the ability to generate names for far more complex structures containing chains, rings and combinations thereof. However, this comes at a price. The parent names are given, they cannot be inferred.
 |
 |
| (f) | (g) |
|---|
|
For example, we can think of baclofen (f) as derived from the parent hydride butane (g) and name it substitutively as 4-amino-3-(4-chlorophenyl)butanoic acid, while chlorquinaldol (h) has the parent hydride quinoline (i) and can be named 5,7-dichloro-2-methylquinolin-8-ol.
 |
 |
| (h) | (i) |
|---|
|
Looking at the names of (f) and (h), we can note a few more “unguessable” features of substitutive nomenclature. First, in order to indicate the points of attachment of the substituents, we need to know how to number the atoms in the parent hydrides. Arabic numerals that show the position of a substituent in a parent structure are known as locants. For example, in the structure (h) the methyl group is attached at the position 2 of its parent hydride, thus we put the locant 2 in front of methyl. Note that in the structure (f), the substituent at the position 3, viz. 4-chlorophenyl, in its turn contains a locant indicating that the chloro group is attached at the position 4 of the phenyl group.
Second, the names of substituents could be used as either prefixes or suffixes. While there could be one, several or no prefixes, substitutive names can have only one suffix. For instance, in (f) the suffix is -oic acid and in (h) it is -ol. How do we chose the substituent for a suffix? Given that the structure (f) contains both carboxylic (–COOH) and amino (–NH2) groups, why did we use -oic acid and not -amine in its name?
This is because the functional groups are ranked according to their “seniority” in a prescribed order of precedence; the highest-ranking group, also known as the principal group, is cited as a suffix and the other substituents as prefixes. Since –COOH group is more senior than –NH2, (f) is named as a carboxylic acid. I have to add that in contrast to, say, electronegativity scale (used, among other things, to construct compositional names), the order of precedence of characteristic groups does not have any particular physical or chemical basis and is rather arbitrary. Besides, substituents such as halo (–F, –Cl, –Br, –I), nitroso (–NO), nitro (–NO2) and azido (–N3) groups are never cited as suffixes. So there’s no more logic than in alphabetical ordering.
To sum up: substitutive nomenclature is a powerful system allowing to construct the names for very complex structures provided that you know
- names of the parent structures;
- how to number atoms in the parent structures;
- the order of precedence of characteristic groups.
References
- Hellwich, K.-H., Hartshorn, R.M., Yerin, A., Damhus, T. and Hutton, A.T. (2020). Brief guide to the nomenclature of organic chemistry (IUPAC Technical Report). Pure and Applied Chemistry 92, 527—539.
- Favre, H.A. and Powell, W.H. Nomenclature of Organic Chemistry: IUPAC Recommendations 2013 and Preferred IUPAC Names. Royal Society of Chemistry, Cambridge, 2014.







No comments:
Post a Comment