Systematic name formation in chemistry typically happens through compounding, derivation, or mix of both. The semantic modification of a combining form through umlaut-like vowel change as seen in alkanes/alkenes/alkynes appears to be unique. Its origin could be traced to the 1866 publication of August Wilhelm von Hofmann [1]; I probably would never know about it if not for an illuminating blog post by Joe Dixon [2].
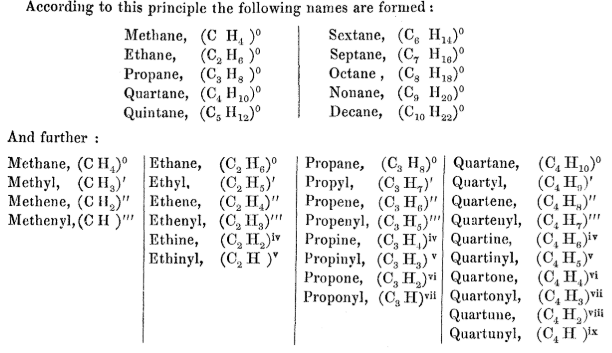
In an extended footnote, Hofmann proposed to call the first ten alkanes as follows: methane, ethane, propane, quartane, quintane, sextane, septane, octane, nonane and decane. For the first three alkanes, Hofmann used by then already established roots:
- ‘meth’, created in 1834 by Jean-Baptiste Dumas and Eugène Péligot from the Greek μέθυ (méthu, “wine”) and ὕλη (húlē, “wood”) to give a more scientificky name to wood alcohol (i.e. methanol) [3]. In his footnote Hoffman equates the word methyl with the group CH3.
- ‘eth’, from ether and that ultimately from Greek αἰθήρ. The name ‘æther’ was given to diethyl ether C2H5–O–C2H5 by Joannes Sigismundus Augustus Frobenius [4]. Justus von Liebig called the group C4H10 ‘ethyl radical’ [5] and gave it a symbol E; ‘æther’ was viewed as an oxide of ‘ethyl radical’. Hoffman, however, gives the word ‘ethyl’ its modern meaning, i.e. equates it with the group C2H5.
- ‘prop’, from propionic acid; the name acide propionique was proposed by Dumas et al. [6] from Greek πρῶτος (prôtos, “first”) and πίων (píōn, “fat”), because it is the first in the series of (true) fatty acids. Hoffman uses the word ‘propyl’ for the group C3H7.
‘Quartane’ did not catch on. Apparently, the root ‘but’, from butyl and that from butyric acid, sounded better — or shall I say, butter? The name acid butérique, from Greek βούτυρος (boúturos, “butter”), was coined in 1817 by Michel Eugène Chevreul [7] who first isolated this “principe odorant extrêmement remarquable” from butter*.
Quintane, sextane, septane did not fare much better: Hofmann’s Latin multipliers had been replaced by Greek ones. Nevertheless, the principle of systematic naming outlined in that footnote, viz. multiplier + ‘ane’, has stayed.
Which brings us to the second point of Hoffman’s proposal: to use the series of vowels “a, e, i, o, u” to indicate the degree of saturation†. Somewhat confusingly for a present-day reader, Hoffman refers to anything beyond ‘ane’ as a “group”, so ‘yl’ corresponds to univalent group, ‘ene’ to bivalent group, ‘enyl’ to trivalent group, ‘ine’ to quadrivalent group, ‘inyl’ to quintivalent group and so on — as you can see, he was consistent in his use of Latin multipliers. It works fine with methane CH4: –CH3 is methyl, –CH2– is ‘methene’ (now methylene or methanediyl) and –CH< is ‘methenyl’ (now methanetriyl). I wonder why there was no ‘methine’ for >CH< (now methanetetrayl). In case of ethane, Hoffman’s terminology stayed practically intact except that ‘ethine’ is called now ‘ethyne’ (all ‘i’s became ‘y’s in English). However, ethene or ethyne in modern sense are not groups but molecules, and both ethenyl and ethynyl are univalent groups. Likewise, propane, propene and propyne are molecules and propanyl, propenyl and propynyl are univalent groups.
Note that in Hoffman’s proposal alkyl groups already have lost the ‘an’ bit: methane → methyl (not methanyl), ethane → ethyl (not ethanyl), propane → propyl (not propanyl) and so on, while the ‘en’ and ‘in’ (as well as ‘on’ and ‘un’) bits are conserved: ethene → ethenyl, ethine → ethinyl, propene → propenyl, propine → propinyl, etc.
Today we identify ‘en’ with double bond and ‘yn’ with triple bond. As there are no known quadruple or quintuple bonds between main-group atoms, there seems to be little use for ‘on’ and ‘un’ in Hoffman’s sense. But if they do exist? The 2012 study proposing the quadruple bond in dicarbon has stirred up much controversy. Even so, the recent discovery of boron–metal quadruple bonds [8, 9] makes me hopeful that “carbon and first-row main elements are open to quadruple bonding” [10]. Well, ‘one’ is out of question as it is used in ketones. But I think we still can employ ‘un’. Could we call C≣C ‘ethune’?
| * | Trivial names such as German Buttersäure, Swedish smörsyra and Russian масляная кислота all mean “butter acid”. |
| † | From phonology point of view, “u, o, a, e, i” would be more logical. |
References
- Hofmann, A.W. (1866) On the action of trichloride of phosphorus on the salts of the aromatic monamines. Proceedings of the Royal Society of London XV, 54—62.
- Dixon, J. Acetylene (and hydrocarbon suffixes). Chemtymology, 3 June 2019.
- Wisniak, J. (2009) Eugène Melchior Peligot. Educación Química 20, 61—69.
- Frobenius, J.S.A. (1730) An account of a spiritus vini æthereus, together with several experiments tried therewith. Philosophical Transactions of the Royal Society of London 36, 283—289.
- Ziegler, F.E. The Composition and Structure of Ether.
- Dumas, J.-B., Malaguti, F. and Leblanc, F. (1847) Sur l’identité des acides métacétonique et butyro-acétique. Comptes rendus hebdomadaires des séances de l’Académie des sciences XXV, 781—784.
- McBride, J.M. Development of systematic names for the simple alkanes.
- Chi, C., Wang, J.-Q., Hu, H.-S., Zhang, Y.-Y., Li, W.-L., Meng, L., Luo, M., Zhou, M. and Jun Li, J. (2019) Quadruple bonding between iron and boron in the BFe(CO)3− complex. Nature Communications 10, 4713.
- Cheung, L.F., Chen, T.-T., Kocheril, G.S., Chen, W.-J., Czekner, J. and Wang, L.-S. (2020) Observation of four-fold boron–metal bonds in RhB(BO–) and RhB. The Journal of Physical Chemistry Letters 11, 659—663.
- Shaik, S., Danovich, D., Braida, B and Hiberty, P.C. (2016) The quadruple bonding in C2 reproduces the properties of the molecule. Chemistry – A European Journal 22, 4116—4128.







No comments:
Post a Comment