What is a covalent bond? We learn in school that it is a chemical bond formed by shared pairs of electrons between atoms. The Gold Book provides a bit more careful definition:
A region of relatively high electron density between nuclei which arises at least partly from sharing of electrons and gives rise to an attractive force and characteristic internuclear distance.
Well, now the textbook definition will have to be changed [1]. In the study published this week in Nature [2], Shimajiri and co-authors
report the isolation of a compound with a one-electron σ-bond between carbon atoms by means of the one-electron oxidation of a hydrocarbon with an elongated C—C single bond. The presence of the C•C one-electron σ-bond (2.921(3) Å at 100 K) was confirmed experimentally by single-crystal X-ray diffraction analysis and Raman spectroscopy, and theoretically by density functional theory calculations.
Cf. the length of single C—C bond in diamond: 1.54 Å. In 2018, Ishigaki et al. [3] reported much longer two-electron C—C bond of 1.806(2) Å in a polycyclic hydrocarbon, dispiro[(dibenzo[a,d]cycloheptatriene)-5,1′-(1′,2′-dihydropyracylene)-2′,5″-(dibenzo[a,d]cycloheptatriene)]* (10c):
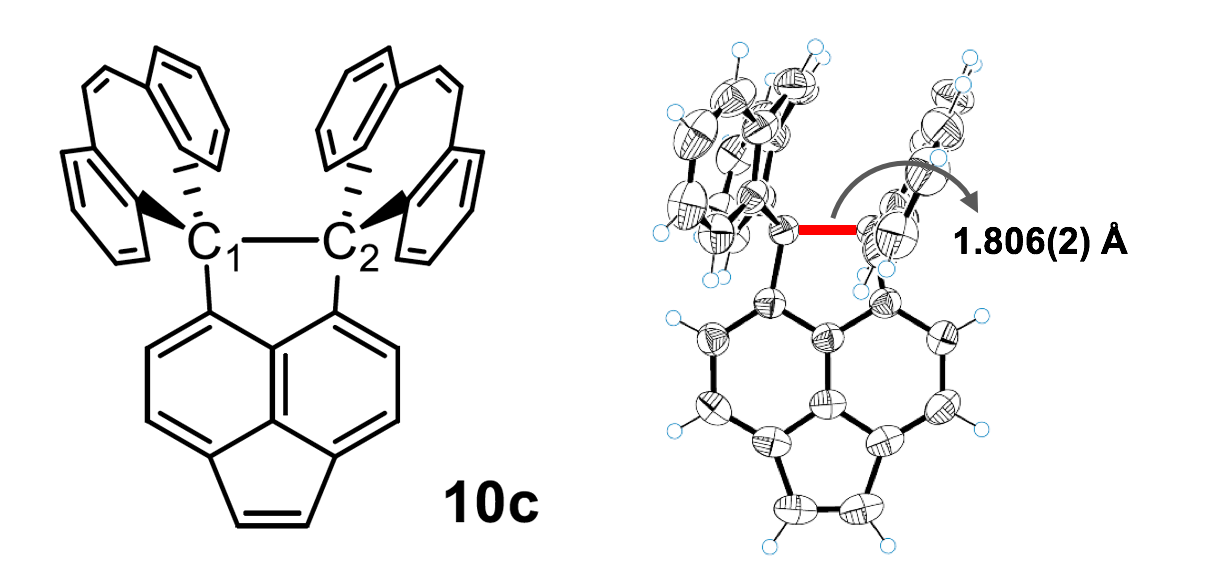
Now the same team took the compound 10c of [3] and crystallised it with iodine. In the resulting stable salt (10c•+)(I3−), the C1—C2 bond lost one electron to I3.
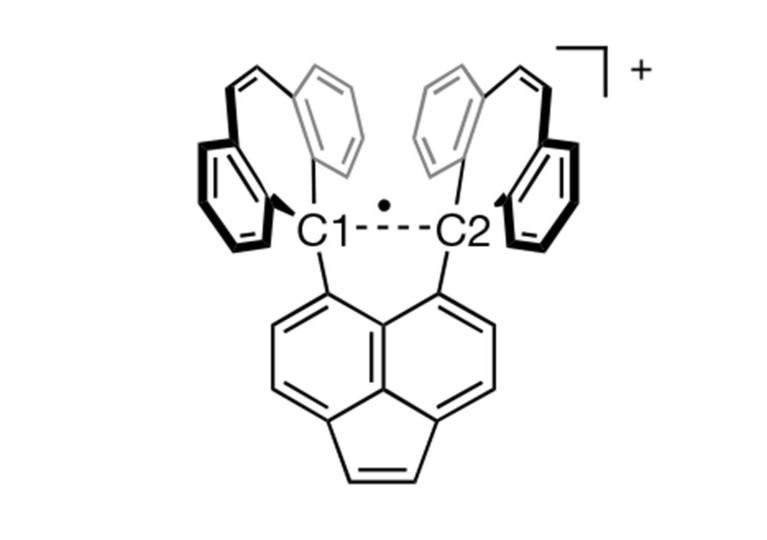
This is not the first one-electron bond observed (see [2] and references 1—4 therein) but the first involving carbon atoms.
The crystal structures of (10c•+)(I3−) are deposited with CCDC, entries 2301032 through 2301039.
| * | This is the name given to the compound by Shimajiri [4]. ‘Pyracylene’ is a trivial name of cyclopent[fg]acenaphthylene. Saturating the bond of the cyclopentane ring, we get 1,2-dihydrocyclopent[fg]acenaphthylene. In spiro nomenclature, the locants of the second component are primed (and of the third component doubly primed, etc.), thus 1′,2′-dihydrocyclopent[fg]acenaphthylene. Therefore, the fully systematic name should be dispiro[(dibenzo[a,d]cycloheptatriene)-5,1′-(1′,2′-dihydrocyclopent[fg]acenaphthylene)-2′,5″-(dibenzo[a,d]cycloheptatriene)]. |
References
- Bourzac, K. (2024) Carbon bond that uses only one electron seen for first time: ‘It will be in the textbooks’. Nature, online ahead of print.
- Shimajiri, T., Kawaguchi, S., Suzuki, T. and Ishigaki, Y. (2024) Direct evidence for a carbon—carbon one-electron σ-bond. Nature, online ahead of print.
- Ishigaki, Y., Shimajiri, T., Takeda, T., Katoono, R. and Suzuki, T. (2018) Longest C—C single bond among neutral hydrocarbons with a bond length beyond 1.8 Å. Chem 4, 795—806.
- Shimajiri, T. (2022) The nature of ultralong C—C bonds: Demonstration of the longest Csp3—Csp3 single bond beyond 1.8 Å and discovery of flexible covalent bonds. Doctoral dissertation, Hokkaido University.







No comments:
Post a Comment