Ferrocene was discovered in 1951 and we still do not know the proper way to draw it. CrossFire example recommends to connect every carbon atom of the ring to the central metal atom. Which is fair enough and will be a valid query for CrossFire Gmelin database. Similarly, both ChEBI and NIST Webbook use decacoordinate iron in ferrocene structure (a). In this representation, all carbon—carbon bonds are single. But, according to IUPAC Recommendations, section GR-1.7.2,
coordination bonds to contiguous atoms (most commonly representing a form of π-bonding) should be drawn to indicate most clearly that special bonding pattern. Depictions that imply a regular covalent bond — and especially, depictions that show a regular covalent bond to each member of a delocalized system — are not acceptable.
In other words, the preferred representation is the one with bicoordinate iron and delocalised bond system (b). The problem with that is there is no agreed (as far as chemoinformaticans are concerned) way to do that, even though solutions for different applications (e.g. for Marvin Sketch) do exist. In MolBase, the coordination number of iron in ferrocene is 6 (and I do remember Mark Winter confirming that this is true). On yet another hand, Beilstein and ChemIDplus databases represent ferrocene as a standalone Fe2+ and two standalone cyclopenta-2,4-dienide anions (c), thus avoiding the question of coordination number altogether. Naturally, the decacoordinate-iron query will not work in Beilstein. (For InChI implications, see this discussion.)
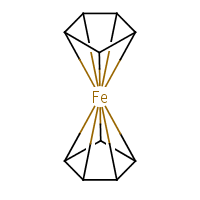 |  |  |
| (a) | (b) | (c) |
|---|







5 comments:
This is a big problem that will only get more troublesome as free, Web-based chemical databases become more widely used and need to interoperate. Ferrocene is one example, but there are many others.
FlexMol is a system that solves this, and many other problems in representing bonding and stereochemistry. A tutorial describes how to use it to represent ferrocene.
I still think that we are overusing 2D diagrams, and certainly those with bad semantics. What are those covalent bonds between all carbons and the iron, or what are those? (I know what they are, but Joe Cheminformatics-Tool does not.) Let's just do 3D. Jmol is free.
FlexMol does indeed to a much better job here than many tools, but, Rich, is it actually hooked with your drawing applet?
Rich, I can't help noticng that your cisplatin example says "central platinum atom surrounded by two chloro ligands and two amino [i.e. NH2] ligands". In fact, there are two ammine [i.e. NH3] ligands in cis- (and trans-) platin. (A lot of databases contain this mistake, e.g. PubChem.) Maybe I miss something here but I do not see how complete FlexMol representation of cisplatin takes the correct number of hydrogens into account.
Egon, I don't think 3D can substitute 2D and vice versa. I would argue that 2D diagram is able to convey some chemical concepts much better than 3D diagram. But what about 3D representation of ferrocene? Will it also have iron with ten covalent bonds, or you suggest we forget about bonds altogether?
Kirill, thanks for catching the typo - it's fixed. Taking a look at the cisplatin example, you'll see that the number of hydrogen atoms is explicitly set at 3 (hydrogens="3"), which FlexMol requires.
I'm not saying FlexMol is perfect - there's lots of room for improvement. But unlike any other language I know of, it does let you unambiguously specify the exact chemical structure you're talking about regardless of how 'unusual' its bonding or stereochemistry.
So far, FlexMol isn't integrated into any drawing tools I'm aware of. The problem is that there's not a lot of agreement about how to do it. The structure (b) is the most common in the literature, but as late as 2007, SciFinder was showing something more like (a).
None of the 2D representations in common use are ideal. For example (b) introduces yet another meaning for wedge bond - something that others have taken pains to define in terms of stereochemisty.
Rich, thank you - I did not know about existence of FlexMol until yesterday. (I clearly was not paying attention, the hydrogen data is there.) My point, however, was about graphical representation and we all agree that there is no ideal representation of ferrocene and similar compounds.
By the way, you just pointed at yet another inconsistency between our two IUPAC recommendations. Indeed, the wedge bond should not be used to indicate perspective, thus examples in GR-1.7.2 should be redrawn with plain bonds. In fact, that would be my preferred way of drawing ferrocene & Co, but it is less common than (b). Yet another way to draw ferrocene is shown here, which, like (c), simply avoids (answering) the question of iron bonding.
Post a Comment