Here’s something one doesn’t see in a chemistry textbook. The recent perspective paper in Nature Chemistry deals with a distinct class of electron-pair bonding called “charge-shift” (CS) bonding, which exists alongside classical covalent and ionic bonding. And in not-so exotic molecules.
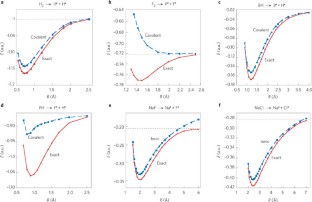
<A> striking example is the difference between H2 and F2; two homonuclear bonds that by all criteria should be classified as covalent bonds, but exhibit fundamental differences. Consider the energy curves (Fig. 1) of the two bonds calculated recently. Figure 1a shows that the H—H bond is indeed covalent; its covalent structure accounts for most of the bonding energy (relative to the ‘exact’ curve). By contrast, for the F—F bond in Fig. 1b, the covalent structure is entirely repulsive, and what determines the bonding energy and the equilibrium distance is the covalent–ionic mixing. This mixing leads to a resonance energy stabilization, which we have termed the ‘charge-shift resonance energy’ (RECS). Thus, despite their apparent similarity, the two bonds are very different; whereas the H—H bond is a true covalent bond, the F—F bond is a CS bond that is completely determined by the RECS quantity.
No less striking example is so-called inverted C—C bond in [1.1.1]propellane (described in a paper from the same group of authors), which “closely resembles the single bond of difluorine”.








No comments:
Post a Comment