The cover of the latest issue of Chemistry International features a fragment of Homenatge als elements (Hommage to the Elements) by the Catalan artist Eugènia Balcells. The display in the atrium of the Physics and Chemistry Library at the University of Barcelona takes the shape of the periodic table where each chemical element is represented by its emission spectrum [1]. According to the artist’s website, it “was born as a counterpoint” to the video installation Freqüències (Frequencies).
The Periodic Table Project at the University of Waterloo, Canada is another work of art,
designed by chemistry students from all Canadian provinces and territories, 20 U.S. states, and 14 countries. It can be viewed online and is available as a printed poster.
Also, as a free app for Apple or Android.
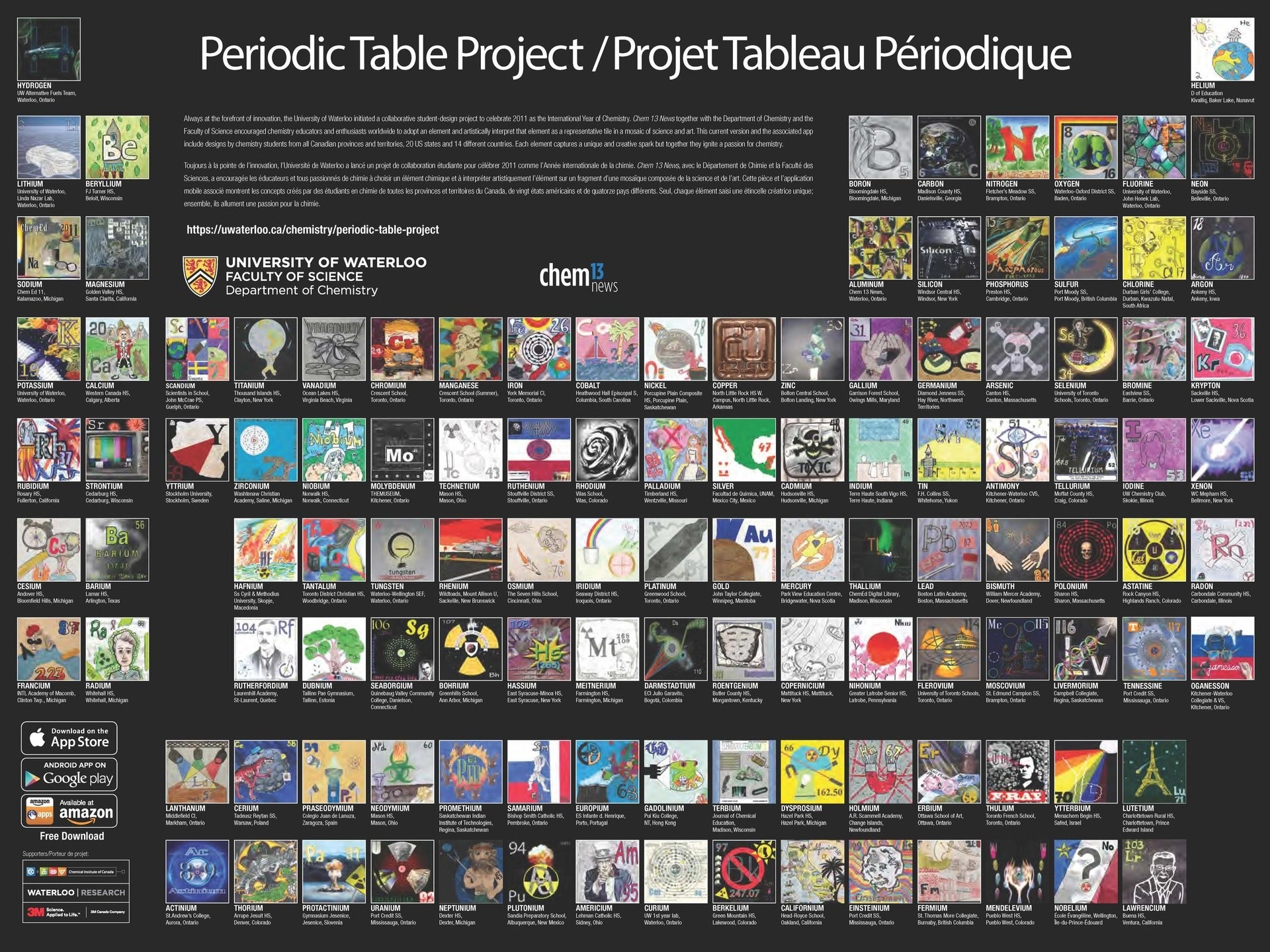
Both the Periodic Table Project and Hommage to the Elements use the medium-long form periodic table. The “IUPAC Periodic Table of the Elements” as published at the back of Chemistry International (in this issue, for the first time it includes flerovium and livermorium) has the same shape. Why the quotes? Because, as a matter of fact, there is no such thing as IUPAC-approved periodic table. Jeffery Leigh wrote three years ago that “there is unlikely to be a definitive IUPAC-recommended form of the periodic table” [2]. In my humble opinion, this is unfortunate that IUPAC refuses to take a position on this matter. Eric Scerri takes a view that “IUPAC should in fact take a stance on the membership of particular groups even if this has not been the practice up to this point” [3]. To illustrate this point, he goes to address the Group 3 question. He argues that the most logical composition of this group is Sc, Y, Lu and Lr (rather than Sc, Y, La and Ac), as shown below.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||||||||||||||
| H | He | ||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 |
In addition to arranging all the elements in a more correct sequence of increasing atomic numbers, the decision to move to a long-form or 32-column table forces the periodic table designer towards just one possible option regarding the question of which elements to place in group 3.
I entirely agree with that. And yet Scerri stops short of proposing that IUPAC should support the 32-column (or “long, long form”, as Leigh put it) periodic table; in fact he explicitly states that he is not suggesting a change of IUPAC policy, viz. that of doing nothing about it. Why? That would be one of the most important and immediately noticeable changes sponsored by IUPAC in decades.
The problem is, sorting out the Group 3 does not resolve the problem how to number the f-block columns. If we stick with 18 groups (blue numbering on the top of the table), that would be really unfair towards the lanthanoids and actinoids. Why don’t we simply number groups from 1 to 32 (red numbers on the bottom of the table)? Sc, Y, Lu and Lr will find themselves in Group 17. So what? It’s not that many people will miss the current Group 17 — nobody really calls these elements anything but “halogens”. And 32 is even more convenient number than 18. I think it’s about time IUPAC took the lead and said how exactly the periodic table should look like.
- Alvarez, S. (2012) An artist’s hommage to the elements. Chemistry International 34, 5.
- Leigh, J. (2009) Periodic tables and IUPAC. Chemistry International 31, 4—6.
- Scerri, E. (2012) Mendeleev’s periodic table is finally completed and what to do about group 3? Chemistry International 34, 28—31.







3 comments:
Thank you for citing my work. I agree with you that ideally IUPAC should also move to a 32-column or long-form periodic table. I just think this should be done in stages not to upset too many cherished beliefs too quickly. Perhaps they could adopt the correct grouping for what is currently called group 3 and then a also consider the move to the 32 column table. Incidentally this will very soon need to be a 50 column table, just as soon as element 121 is synthesized.
ericscerri
see ericscerri.com/ for more on this and related issues
Dear Eric,
thank you for a comment. My main problem is that it is not clear what the IUPAC position is. IMHO it is not good enough to have something published (and widely referred to) as "IUPAC Periodic Table" and then claim that IUPAC in fact does not approve this or any other form. As is the case with any IUPAC recommendations, some will follow it and some will ignore it, but one needs to have a recommendation to start with. (E.g. when IUPAC publishes the New Blue Book there will be PINs, i.e. "preferred IUPAC names". Nobody says they are the only names to be used, but they do set some sort of standard.) And I don't think there will be too many people terribly upset by IUPAC saying what form is preferred. If there are people still wanting to use the short form of periodic table that's fair enough, but it's better that they do it in the knowledge that this form is, officially, not preferred — something that's not the case now.
Thank you for your comment.
This is precisely my point. IUPAC does not take an official position on the grouping of elements even if some people mistakenly claim that it does. I am in the process of putting together an official proposal to IUPAC. I welcome any input and comment.
please also see
ericscerri.com/
Post a Comment