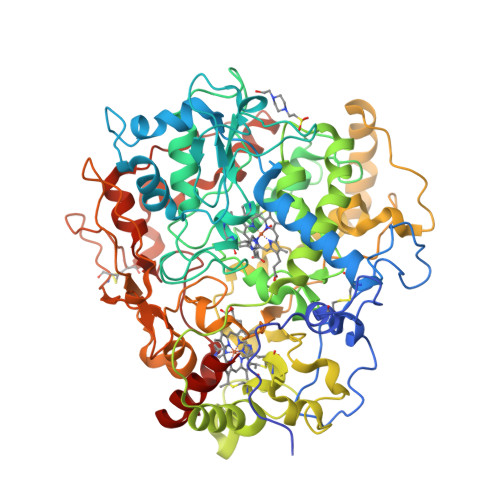
To date, two types of enzymes that are responsible for primary attack of polyisoprene in rubber-degrading microorganisms have been identified [1]. One is the latex clearing protein (Lcp), first isolated from Streptomyces sp., which does not have any metal ions or cofactors [2]. The other is the rubber oxygenase RoxA of Xanthomonas sp., a dihaem c-type cytochrome that cleaves cis-1,4-polyisoprene, the main constituent of natural rubber, to 12-oxo-4,8-dimethyltrideca-4,8-diene-1-al [3, 4]. The crystal structure of RoxA, solved at 1.8 Å resolution, was released today [5].
- Birke, J., Hambsch, N., Schmitt, G., Altenbuchner, J. and Jendrossek, D. (2012) Phe317 is essential for rubber oxygenase RoxA activity. Applied and Environmental Microbiology 78, 7876—7883.
- Rose, K., Tenberge, K.B. and Steinbüchel, A. (2005) Identification and characterization of genes from Streptomyces sp. strain K30 responsible for clear zone formation on natural rubber latex and poly(cis-1,4-isoprene) rubber degradation. Biomacromolecules 6, 180—188.
- Braaz, R., Fischer, P. and Jendrossek, D. (2004) Novel type of heme-dependent oxygenase catalyzes oxidative cleavage of rubber (poly-cis-1,4-isoprene). Applied and Environmental Microbiology 70, 7388—7395.
- Schmitt, G., Seiffert, G., Kroneck, P.M.H., Braaz, R. and Jendrossek, D. (2010) Spectroscopic properties of rubber oxygenase RoxA from Xanthomonas sp., a new type of dihaem dioxygenase. Microbiology 156, 2537—2548.
- PDB:4B2N







No comments:
Post a Comment