In a number of IUPAC publications, the entities that are referred to as “stems” include
- Latin stems such as ‘argent’, ‘aur’, ‘cupr’, ‘ferr’, etc. used before ‘ide’ or ‘ate’ in anion names [1];
- Stem name ‘carotene’ in nomenclature of carotenoids [2, rule 2];
- Stem ‘calci-’ in nomenclature of vitamin D [3];
- Stem ‘retin-’ in nomenclature of retinoids [4];
- In carbohydrate nomenclature, stem names that designate the chain length of the sugar, e.g. ‘pent-’, ‘hex-’, ‘hept-’ etc. [5];
- Stems such as ‘irene’, ‘irane’, ‘epine’ etc. in Hantzsch-Widman (H-W) nomenclature [6];
- Stem name ‘phosphatidic acid’ [7].
Before we go any further, we have to distinguish between the terms “root”, “stem” and “base”, which are often used interchangeably even in linguistic literature. According to Laurie Bauer [8],
A root is a form which is not further analysable, either in terms of derivational or inflectional morphology. It is that part of word-form that remains when all inflectional and derivational affixes have been removed.
Here, I am going to stick to Bauer’s approach with one important modification: in addition to “all inflectional and derivational affixes”, I’d like to mention that other roots also have to be removed. For example, sulfur, sulfate, sulfide, sulfite, sulfo, sulfonic, sulfuric, peroxysulfate etc. all contain the root ‘sulf’. Most of the IUPAC examples above are single roots.
A stem is of concern only when dealing with inflectional morphology.
In other words, stem is the part of a word that contains no inflectional affixes. Minimally, stem consists of a root. For example, salt, salts, salted and salting all contain the stem salt, which in this case is identical with the root. Stems can also include derivational affixes, for example desalt = de- (prefix) + salt (root) or salty = salt (root) + -y (suffix), and may comprise more than one root, as in saltpetre or saltwater.
As there is very little inflection in English in general and in English chemical terminology in particular, we do not have much need to talk about “stems”.
A base is any form to which affixes of any kind can be added.
Again, I’d like to add other roots to Bauer’s definition. The H-W “stems” like ‘irene’ are always used in conjunction with element roots such as ‘az’, ‘bor’, ‘ox’, ‘sil’ and so on. So they are not really stems. They are not roots either as they are “further analysable”, for example, in ‘irene’ the root ‘ir’ means three-member cycle, ‘en’ indicates unsaturation and (optional) terminal ‘-e’ is the ending. The term “base” fits the bill here:
 | |||||
| word: | oxirene | ||||
|---|---|---|---|---|---|
| stem: | oxiren | ending: | e | ||
| base: | ox | iren | |||
| root: | ox | ir | en | ||
I understand the potential reluctance of chemists to use the word “base” as it has a few other meanings. However, when we are talking about the name formation, it’s hard to confuse it with other “bases”.
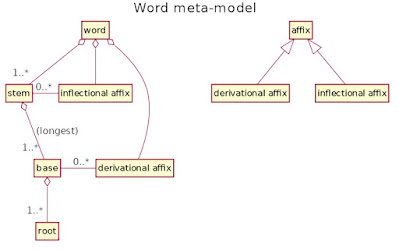 |
| The word meta-model diagram courtesy of Heikki Lehväslaiho |
Lastly, it should be obvious that ‘phosphatidic acid’ [7] can be neither “stem” nor “base” for a simple reason that it’s not a part of a word but a noun phrase that consists of two separate words. In case of ‘phosphatidic’, its stem is identical to the complete word while containing a Russian-dollful of bases, each of them further modifiable with affixes and roots: ‘phosph’ (e.g. phosphane), ‘phosphat’ (e.g. triphosphate), ‘phosphatid’ (e.g. phosphatidylcholine), ‘phosphatidic’ (e.g. diphosphatidic). As for ‘acid’, its root, base and stem are all identical to the complete word.
| phrase: | phosphatidic acid | |
|---|---|---|
| word: | phosphatidic | acid |
| stem: | phosphatidic | acid |
| base: | phosph / phosphat / phosphatid / phosphatidic | acid |
| root: | phosph | acid |
References
- Hartshorn, R.M., Hellwich, K.-H., Yerin, A., Damhus, T. and Hutton, A.T. (2015) Brief guide to the nomenclature of inorganic chemistry (IUPAC technical report). Pure and Applied Chemistry 87, 1039—1049.
- IUPAC and IUPAC-IUB (1975) Nomenclature of carotenoids (rules approved 1974). Pure and Applied Chemistry 41, 405—431.
- IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) (1982) Nomenclature of vitamin D (Recommendations 1981). Pure and Applied Chemistry 54, 1511—1516.
- IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) (1983) Nomenclature of retinoids. Recommendations 1981. Pure and Applied Chemistry 55, 721—726.
- McNaught, A.D. (1996) Nomenclature of carbohydrates (IUPAC recommendations 1996). Pure and Applied Chemistry 68, 1919—2008. 2-Carb-17. Unsaturated monosaccharides.
- Powell, W.H. (1983) Revision of the extended Hantzsch-Widman system of nomenclature for heteromonocycles (Recommendations 1982). Pure and Applied Chemistry 55, 409—416.
- IUPAC-IUB Commission on Biochemical Nomenclature (CBN) (1978) Nomenclature of lipids. Recommendations, 1976. Biochem. J. 171, 21—35. Supplement: Derivatives of phosphatidic acid.
- Bauer, L. (1983) English Word-Formation. Cambridge University Press, 1983, pp. 20—22.







No comments:
Post a Comment