Are you tired of carbocycles? Let’s have some ring diversity, I say.
Structures that contain two or more different elements in a ring are called heterocyclic. Perhaps because “heteroatom” is really an organic chemistry concept, the word “heterocycle” is commonly (mis)understood as “organic heterocycle”, that is, a carbocycle where at least one carbon atom is replaced by an heteroatom. I blame organic chemists for that.
For a small number of five- and six-membered organic heterocycles the trival names are retained to be used as parent hydride names. Note that, although “trivial” in chemical parlance means “non-systematic”, there is a system to most of those names. For instance, we can see that imidazolidine (a) is a fully saturated version of 1H-imidazole (b):
 |
 |
| (a) | (b) |
|---|
|
Similar relationships can be observed between pyrrolidine and pyrrole, pyrazolidine and pyrazole. On the other hand, thiomorpholine (c) is morpholine (d) where an oxygen atom has been replaced by a sulfur atom, in accordance with the standard use of ‘thio’ in chemical nomenclature. Selenophene and tellurophene, predictably enough, are selenium and tellurium analogues of thiophene. So, some of these “trivial” names are in fact “semi-systematic” (or “semi-trivial”, whatever you prefer). Still, that’s about as far as you can get. There is a whole wide world of heterocycles and we need to be able to name them.
 |
 |
| (c) | (d) |
|---|
|
There are two main methods of naming heterocycles systematically. One is to use skeletal replacement nomenclature that we are already familiar with. Another one is the extended Hantzsch-Widman system [1].
This system is used to name saturated and mancude monocycles up to ten ring members. It is named after Arthur Rudolf Hantzsch and Oskar Widman, who independently introduced methods for naming five- and six-membered nitrogen-containing monocycles [2, 3]. It would be more fair to call it Hantzsch-Weber-Widman system, but life is not fair and sadly the name of Hantzsch’s coauthor was all but forgotten — to the degree I can’t find any information on him, including his first names. On the other hand, this nomenclature system is sometimes referred to as Hantzsch-Widman-Patterson system, in honour of Austin McDowell Patterson (1876—1956).
A Hantzsch-Widman (H-W) name minimally consists of at least one ‘a’ “prefix” followed by a “stem” [1] or “ending” [4, p. 96] that indicate the size and saturation of the ring. We know by now that none of these word parts qualifies to be either prefix or ending because of their high semantic load. As mentioned earlier, skeletal replacement “prefixes” such as ‘aza’, ‘bora’, or ‘phospha’ are combining forms composed of roots ‘az’, ‘bor’, ‘phosph’, respectively, plus the functional morpheme ‘a’. The H-W “stems” are also combining forms composed of roots ‘epin’, ‘iren’, ‘olan’ etc. followed by ending ‘e’ which, in any case, is optional [1, p. 413, note b]. Additionally, H-W names may contain multipliers (‘di’, ‘tri’, etc.) and locants. The H-W ring roots are summarised in the table below.
| Order | Last-cited heteroatom | Mancude | Saturated |
|---|---|---|---|
| 3 | irene / irine* | irane / iridine† | |
| 4 | ete | etane / etidine† | |
| 5 | ole | olane / olidine† | |
| 6(A) | O, S, Se, Te, Po, Bi | ine | ane |
| 6(B) | N, Si, Ge, Sn, Pb | ine | inane |
| 6(C) | F, Cl, Br, I, P, As, Sb, B, Al, Ga, In, Tl | inine | inane |
| 7 | epine | epane | |
| 8 | ocine | ocane | |
| 9 | onine | onane | |
| 10 | ecine | ecane | |
| * For rings with nitrogen as only heteroatom | |||
| † For rings containing nitrogen | |||
You may remember that the substitutive names of carbocycles, except for benzene and benzene ring-containing structures, are based on the names of cycloalkanes, i.e. saturated parents. In the H-W system we have a choice. To name a saturated ring, we choose saturated root; to name an unsaturated ring, we pick mancude root. It’s best to see how it works looking at the examples:
As you can see from these examples, H-W names are significantly shorter tham the corresponding replacement names.
A few observations.
Upon combining with a corresponding H-W root, a replacement (‘a’) term loses its characteristic final ‘a’ “when followed by a vowel” [1, Rule RB-1.2]‡. Since all H-W roots begin with a vowel, all replacement terms immediately preceding a H-W root lose the final ‘a’: azetidine (not azaetidine), 1,3-dithiolane (not 1,3-dithiaolane), 1,3,5-trioxane (not 1,3,5-trioxaane) and so on. This also happens if a replacement form is followed by another replacement term that begins with a vowel, as in oxazirene (not oxaazairene). By comparison, in skeletal replacement nomenclature the final ‘a’ of the replacement term is never omitted, whether the structure is acyclic or cyclic, as in 3,6,9,12,15,18-hexaoxaicosane or 1-oxa-2-azacycloprop-2-ene.
A heteromonocycle with two or more different heteroatoms is named according to the priority sequence (1):
F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl | (1) |
|---|
thus oxazirene (not azoxirene), thiadiazole (not diazathiole), etc.
Some mancude rings contain saturated atoms. To avoid ambiguity in the names, such atoms are specified using the indicated hydrogen descriptor, which consists of the locant followed by a hydrogen symbol, as in 1H-imidazole (b) (to distinguish it from 4H-imidazole) or in 1H-arsole (because 2H- and 3H-tautomers are also possible). Unfortunately, indicated hydrogen, like most chemical descriptors, compromises pronounceability of resulting names.
What about unsaturated rings that are not mancude? In this case, one has to either start with mancude parent name and modify it additively with ‘dihydro’, ‘tetrahydro’, etc., or start with saturated parent name and modify it subtractively with ‘didehydro’, ‘tetradehydro’, etc. The first method is “usually preferred” [1, Rule RB-1.5], although I personally would go for a method that results in a simpler name, avoiding, if possible, indicated hydrogen. For example, the structure (e) could be named either 2,3-dihydro-1H-1,2,4-triazole (additively) or 3,4-didehydro-1,2,4-triazolidine (subtractively).
 |
| (e) |
|---|
|
Although the H-W system was developed for organic heterocycles, i.e. default atom to be replaced is carbon, the ring to be named does not need to contain any carbon:
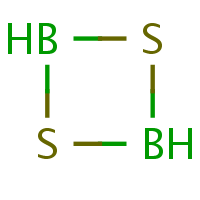 |
 |
 |
| (f) | (g) | (h) |
|---|
|
I have to say that H-W names for the structures (f) — (h) somehow don’t appeal to me. They just have too many locants for my taste. Thankfully, there is yet another naming method “for the special case of saturated rings of two alternating skeletal atoms” [4, p. 96]. Here, the name for a cycle that contains n pairs of skeletal atoms –X–Y– consists of ‘cyclo’, followed by a multiplier for n, followed by the replacement terms for X and Y, followed by ‘ane’. For reasons unknown, here the replacement terms are cited in the reverse of the order of the priority sequence (1). For want of a better name, I call this latter method NASA (for “n alternating skeletal atoms”). One does not have to be an expert to see that cyclotetrasiloxane (note Si > O!) is a much more elegant name for (h) than 1,3,5,7,2,4,6,8-tetroxatetrasilocane (here O > Si).
Still, the extended H-W system could be used to name some inorganic heterocycles or, indeed, inorganic homocycles:
 |
 |
 |
| (i) | (j) | (k) |
|---|
|
The only kind of cycles that are not named with H-W system are carbocycles, although I don’t see why one cannot do that. For once, the name ‘ocine’ is much shorter than ‘cycloocta-1,3,5,7-tetraene’ and even ‘[8]annulene’.
To sum up:
- The extended Hantzsch-Widman system can be used to name organic heterocycles, inorganic heterocycles, and inorganic homocycles up to ten members, with any number of heteroatoms from the list (1);
- A H-W name consists of two parts: replacement (‘a’) term(s) followed by a H-W ring root;
- In the special case of saturated rings of two alternating skeletal atoms, another naming method (which I call NASA) can be employed;
- For ring sizes greater than ten we have no choice but use skeletal replacement nomenclature.
| ‡ | In a number of nomenclature publications, including [1] and [4], this vowel loss is referred to as “elision”. I need to do more research on the topic because I am not convinced that this linguistic term is being used correctly. Watch this space. |
References
- Powell, W.H. (1983) Revision of the extended Hantzsch-Widman system of nomenclature for heteromonocycles. Pure and Applied Chemistry 55, 409—416.
- Hantzsch, A. and Weber, J.H. (1887) Ueber Verbindungen des Thiazols (Pyridins der Thiophenreihe). Berichte der Deutschen Chemischen Gesellschaft 20, 3118–3232.
- Widman, O. (1888) Zur Nomenclatur der Verbindungen, welche Stickstoffkerne enthalten. Journal für praktische Chemie 38, 185—201.
- Connelly, N.G., Hartshorn R.M., Damhus, T. and Hutton, A.T. Nomenclature of Inorganic Chemistry: IUPAC Recommendations 2005. Royal Society of Chemistry, Cambridge, 2005.


















No comments:
Post a Comment