What is the best way to name the structure (a)?
 |
| (a) |
|---|
|
The general naming method is skeletal replacement applied to the corresponding carbocyclic parent hydride, in our case cyclononane, thus 1,4,7-trioxacyclononane. Or we can use extended Hantzsch-Widman (H-W) system and call it 1,4,7-trioxonane. For rings with up to ten members, the H-W names are preferred [1, p. 96].
What about the structures (b)—(d) then? Since all of these rings have more than ten members, we cannot use H-W system, so we have to give them replacement names: 1,4,7,10-tetraoxacyclododecane (b), 1,4,7,10,13-pentaoxacyclopentadecane (c), 1,4,7,10,13,16-hexaoxacyclooctadecane (d). Rather long, completely unambiguous, and very boring. The situation is akin to that with annulenes: as the rings grow in size, their systematic names also grow longer without reflecting the simple fact that all these structures consist of the same repeating unit.
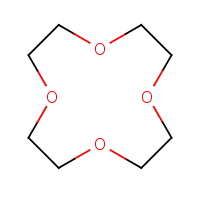 |
 |
 |
| (b) | (c) | (d) |
|---|
|
The structures like (a)—(d) are known as crown ethers. They could be defined as macrocycles consisting of several oxyethylene (–CH2–CH2–O–) groups. Adopting the IUPAC nomenclature for cyclic organic macromolecules [2] which recommends names of the form ‘cyclo[poly(constitutional repeating unit)]’, we can assign the names cyclo[tri(oxyethylene)] to (a), cyclo[tetra(oxyethylene)] to (b) and so on*. That’s better, isn’t it? No locants have to be cited, which is always a good news.
Another nomenclature system was proposed by the discoverer of crown ethers himself, Charles J. Pedersen (1904—1989) in his seminal 1967 paper [3]. He came up with short names of the form m-crown-n, where m is the ring size and n is the number of oxygen atoms in the ring. In some variants of Pedersen names, the first number is taken in square brackets, as in [18]crown-6 for (d), perhaps by analogy with annulenes, perhaps to stress that this number is not a locant (in Pedersen system, no locants are needed for unsubstituted crowns). For any crown ether that is cyclo[n(oxyethylene)] it is easy to reconstruct the structure from the name like m-crown-n. Moreover, since m = 3n, one can argue that even this short name is redundant; e.g., for the structure (d) either 18-crown or crown-6 would suffice. I would go for the latter since the number of oxygen atoms is more important.
Problems arise when we try to use the simple system to name the molecules that deviate from cyclo[n(oxyethylene)] formula. Not satisfied with Pedersen names, Vögtle and Weber developed a more comprehensive nomenclature in an attempt to cover cryptands and podands in addition to crowns [4, 5]. In their system, crowns are named ‘coronands’; for instance, the proposed name for (d) is 18<O6coronand-6>†. Further, Donald James Cram (1919—2001), who would share the 1987 Nobel Prize in Chemistry with Pedersen and Jean-Marie Lehn, suggested to shorten ‘coronands’ to ‘corands’ [6]. Perhaps he was driven by desire to bring it in line with two-syllable ‘cryptands’, ‘podands’ and his own ‘spherands’, but, in doing so, he created a nonsensical term that is still longer than ‘crown’.
Now let’s have a look at the first ever crown discovered by Pedersen (e):
 |
| (e) |
|---|
|
Pedersen named this molecule dibenzo-18-crown-6. Now there are three possible ways to fuse two benzene rings with 18-crown-6 (d) and so Pedersen name may appear ambiguous. Not that he wasn’t aware of that. He wrote [3]:
The placements of the hydrocarbon rings and the oxygen atoms are as symmetrical as possible in most cases, and the exceptions are indicated by asym.
So there. Since there is no asym in the name, dibenzo-18-crown-6 must correspond to the most symmetrical isomer. Compare that with Vögtle-Weber’s 18<O6(1,2)benzeno.22.(1,2)benzeno.22coronand-6> which is not significantly shorter than “cumbersome and less illustrative” [5, p. 3] IUPAC name, dibenzo[b,k][1,4,7,10,13,16]hexaoxacyclooctadecane, and you will understand why chemists still stick to Pedersen names in spite of all their limitations.
Crowns can contain heteroatoms other than oxygen:
 |
 |
 |
| (f) | (g) | (h) |
|---|
|
Sulfur-containing crowns are variously referred to as ‘thiacrowns’, ‘crown thioethers’ [7] or ‘coronand sulfides’ [5, p. 7], and nitrogen-containing ones as ‘azacrowns’, ‘crown amines’, or ‘coronand amines’ [5, p. 7]. For crowns with only one type of heteroatom, modified Pedersen names like trithia-9-crown-3 for (f) or tetraaza-12-crown-4 for (g) have been used in the literature although it would be more correct to call them trithio-9-crown-3 and tetraimino-12-crown-4, respectively‡. We can also create “macrocyclic” names such as cyclo[tri(sulfanediylethylene)] for (f) and cyclo[tetra(iminoethylene)] for (g). As soon another type of heteroatom makes its way into a crown, such relatively simple names are out of question. To avoid ambiguity, we’ve got to use locants, so I guess we’re better off with old H-W or replacement names. To quote Gokel & Fedders [8],
For the time being, the nomenclature of crowns and other heteromacrocycles will likely remain imperfect due to the inherent complexity of the structures. Nevertheless, standard heterocycle naming practices should be applied but often have not been. For example, crowns containing O, S, and/or N should be numbered starting with O rather than one of the atoms differentiating the compound from a simple crown.
So we can name (h) 4,7,13,16-tetraphospha-18-crown-6, which is still shorter than 1,10-dioxa-4,7,13,16-tetraphosphacyclooctadecane. Another unambiguous alternative is a Vögtle-Weber-type name which I suppose is 18<OPPOPP-coronand-6> (where ‘OPPOPP’ is the sequence of heteroatoms), but don’t quote me on that. Meanwhile, IUPAC recommends abbreviated ligand names such as [9]aneS3 (f), [12]aneN4 (g) and [18]aneP4O2 (h) [1, Table VII, p. 261].
| * | The recommendations [2, p. 209] explicitly state: “The same format can be used for oligomer nomenclature, e.g., cyclo[tetra(constitutional repeating unit)].” So I am not making this up. |
| † | Specified by Weber and Vögtle thus [5]:
The number preceding the angular brackets ‘< >’ indicates the ring size. In the presence of aromatic and heteroaromatic units in the ring, the shortest way to the next donor atom is considered. The angular brackets contain in the order given: 1) donor heteroatoms expressed by elemental symbols; 2) bridges, i.e. C–C chains between the donor atoms, denoted by numbers which correspond to the bridging C-atoms, bridge units like aromatic nuclei or more complex groups (position marked in round brackets). The designation ‘2’ for ethano, the most common bridge, is omitted if only this kind of bridge unit is present or if such a procedure does not curtail the clarity of the structure (cf. 18<O6coronand-6>); 3) the class name (e.g. coronand), and 4) the total number of donor heteroatoms. |
| ‡ | In general and organic chemical nomenclature, ‘thio’ means replacement of any oxygen atom with a sulfur atom. In organic nomenclature, ‘imino’ refers to replacement of an endocyclic oxygen atom by a nitrogen atom. On the other hand, in skeletal replacement nomenclature, ‘aza’ and ‘thia’ denote replacement of carbon with nitrogen and sulfur, respectively. |
References
- Connelly, N.G., Hartshorn R.M., Damhus, T. and Hutton, A.T. Nomenclature of Inorganic Chemistry: IUPAC Recommendations 2005. Royal Society of Chemistry, Cambridge, 2005.
- Mormann, W. and Hellwich, K.-H. (2008) Structure-based nomenclature for cyclic organic macromolecules (IUPAC Recommendations 2008). Pure and Applied Chemistry 80, 201—232.
- Pedersen, C.J. (1967) Cyclic polyethers and their complexes with metal salts. Journal of the American Chemical Society 89, 7017—7036.
- Weber, E. and Vögtle, F. (1980) Classification and nomenclature of coronands, cryptands, podands, and of their complexes. Inorganica Chimica Acta 45, L65—L67.
- Weber, E. and Vögtle, F. Crown-type compounds — An introductory overview. In: Vögtle, F. and Weber, E. (eds.) Host Guest Complex Chemistry / Macrocycles. Springer-Verlag, Berlin, Heidelberg, 1985, pp. 1—41.
- Cram, D.J. (1986) Preorganization — From solvents to spherands. Angewandte Chemie International Edition in English 25, 1039—1057.
- Cooper, C.R. (1988) Crown thioether chemistry. Accounts of Chemical Research 21, 141—146.
- Gokel, G.W. and Fedders, M.F. (1996) Ten-membered rings or larger with one or more nitrogen and oxygen and/or sulfur atoms. Comprehensive Heterocyclic Chemistry II vol. 9, 863—892.







No comments:
Post a Comment