Now let us have a look at monocyclic hydrocarbons, starting with cycloalkanes. By the way, I think this term is a bit misleading: cycloalkanes indeed contain cycles but are not alkanes because these latter, by definition, are acyclic. Gold Book defines cycloalkanes as “saturated monocyclic hydrocarbons (with or without side chains)”, where “side chains” are alkyl groups. The general molecular formula of cycloalkanes, with or without side chains, is CnH2n. I wish there was an elegant collective term for cycloalkanes-with-no-side-chains, or unsubstituted cycloalkanes, because only this subset of cycloalkanes can be used as parent hydrides in systematic organic nomenclature; I am not aware of any. Here, I will refer to unsubstituted cycloalkanes as ‘cycloalkane parents’*.
Using the graph theory language, we can say that every cycloalkane is a (hydrogen-depleted) unicyclic graph or 1-tree in which all vertices are carbon atoms, while cycloalkane parents are cycle graphs.
And so on.
The names of cycloalkanes, just like those of alkanes, only imply their carbon and hydrogen composition, which again could be contrasted with the names of their silicon analogues. For example, the name ‘cyclopentasilane’ is easily analysed in terms of combining forms ‘cycl(o)’ = cyclic, ‘pent(a)’ = five, ‘sil’ = silicon and ‘an’ = saturated hydride, i.e. “cyclic hydride containing five silicon atoms”. On the other hand, ‘cyclopentane’ simply means “cyclic saturated hydride containing five non-hydrogen atoms”. Also, like n-alkyl group names, the cycloalkyl group names lack the ‘an’ bit: cyclopropane → cyclopropyl (not cyclopropanyl), cyclobutane → cyclobutyl (not cyclobutanyl), cyclopentane → cyclopentyl (not cyclopentanyl), etc., whicle their silicon analogues will retain ‘an’ as in cyclopentasilane → cyclopentasilanyl.
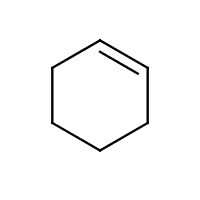
Since there are no terminal vertices, no locants are needed for a single cycloalkane parent modification, be it a substitution or unsaturation. For example, we name the structure (a) methylcyclohexane, not 1-methylcyclohexane; the structure (b) cyclohexene, not cyclohex-1-ene; and (c) cycloundecyne, not cycloundec-1-yne:
 |
 |
 |
| (a) | (b) | (c) |
|---|
|
As soon as another modification is introduced into a ring though, we do need locants to differentiate between isomers. For instance, the structure (d) is named 1,4-dimethylcyclohexane (because both 1,2-dimethylcyclohexane and 1,3-dimethylcyclohexane also exist); (e) is called cyclohexa-1,3-diene (both cyclohexa-1,2-diene and cyclohexa-1,4-diene are also possible); and (f), cyclooctadeca-1,3,5,7,9,11,13,15,17-nonayne†.
 |
 |
 |
| (d) | (e) | (f) |
|---|
|
Speaking about unsaturation: according to Gold Book,
Unsaturated monocyclic hydrocarbons having one endocyclic double or one triple bond are called cycloalkenes and cycloalkynes, respectively. Those having more than one such multiple bond are cycloalkadienes, cycloalkatrienes, etc. The inclusive terms for any cyclic hydrocarbons having any number of such multiple bonds are cyclic olefins or cyclic acetylenes.
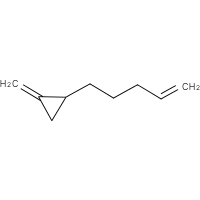
Nothing specific is said about the side chains, nevertheless it is understood that unsaturation outside the ring does not convert a cycloalkane into a cycloalkene or cycloalkyne. Thus, the structure (g) most likely is a cycloalkene while (h) definitely isn’t one. But what is it?
 |
 |
| (g) | (h) |
|---|
|
Gold Book defines alicyclic compounds as
Aliphatic compounds having a carbocyclic ring structure which may be saturated or unsaturated, but may not be a benzenoid or other aromatic system.
The second part of the definition appears to be redundadnt since aliphatic compounds are already said to be
Acyclic or cyclic, saturated or unsaturated carbon compounds, excluding aromatic compounds.
Nothing prevents alicyclic structures from having more than one ring provided they are not aromatic; however, we’ll leave polycyclic structures for another time. I suppose ‘alicyclic monocycles’ or ‘monoalicyclic structures’ could be an umbrella term for all structures in this post.
Naming monoalicyclic hydrocarbons is straightforward: rings are always senior to chains irrespectively of their length [1]. Thus, we name the structure (i) 1-methyl-4-(6-methylheptan-2-yl)cyclohexane, not 2-methyl-6-(4-methylcyclohexyl)heptane‡.
 |
| (i) |
|---|
|
| * | I came across the term ‘alkylcycloalkanes’ which explicitly refers to cycloalkanes with side chains; however, if we accept this term, we should reserve the use of ‘cycloalkanes’ for cycloalkane parents only. |
| † | Although cyclo[18]carbon (f) can be systematically named as a cycloalkapolyyne, it hardly qualifies to be among cyclic hydrocarbons for the simple reason that it does not have any hydrogen. |
| ‡ | In earlier recommendations, seniority depended on number of atoms. If there were more atoms in the chain than in the ring, the hydrocarbon name was based on the chain name [2], thus 2-methyl-6-(4-methylcyclohexyl)heptane would be preferred to 1-methyl-4-(6-methylheptan-2-yl)cyclohexane. |
References
- Hellwich, K.-H., Hartshorn, R.M., Yerin, A., Damhus, T. and Hutton, A.T. (2020). Brief guide to the nomenclature of organic chemistry (IUPAC Technical Report). Pure and Applied Chemistry 92, 527—539.
- Panico, R., Powell, W.H. and Richer, J.-C. A Guide to IUPAC Nomenclature of Organic Compounds (Recommendations 1993). Blackwell Science, 1993.













No comments:
Post a Comment