Can we expand the parent hydride naming philosophy much beyond organic chemistry? Not going too far, let’s have a peek at carbon’s immediate neighbour in the periodic table, boron.
 |
| (a) |
|---|
|
The mononuclear hydride (a) is systematically named ‘borane’ while neutral boron hydrides as a class are called boranes.
.png) |
.png) |
 |
| (b) | (c) | (d) |
|---|
|
Ignoring for a moment the number of hydrogen atoms, the structures (b)—(d) can be thought of as boron analogues of ethane (dicarbane*), propane (tricarbane*) and cyclobutane (cyclotetracarbane*), respectively. Logically enough, we can name (b) diborane, (c) triborane and (d) cyclotetraborane. Right?
And now the moment’s gone. Boron hydride nomenclature, in contrast to that of hydrocarbons, explicitly specifies the number of hydrogen atoms in the molecule [1—3]. This is done by placing the corresponding Arabic numeral in parentheses immediately following the name†. Thus, (b) will be diborane(4), (c) triborane(5) and (d) cyclotetraborane(4). The structure (c) could be also named catena-triborane(5), the descriptor catena emphasising that it’s a chain [2, IR-6.2.3.2, p. 92]. As for (d), the Blue Book gives the Hantzsch-Widman name — back to implicit hydrogen! — ‘tetraboretane’ as preselected [4, P-68.1.1.3.2].
 |
| (e) |
|---|
|
Since the name of the group –BH2 is boranyl, the molecule (e) could be named substitutively triboranylborane. However, the IUPAC name is 2-boranyltriborane(5). Although (e) contains six hydrogens, the numeral in parentheses is 5. Why? Because this name is based on the parent hydride triborane(5) (c), substituted by one boranyl group at the position 2.‡
Why do we even have to specify the number of hydrogens? Consider the structure (f):
 |
| (f) |
|---|
|
It is also a ‘diborane’ and it is very different from diborane(4) (b). Not only does (f) contain two tetravalent boron atoms, but it also has two bridging hydrogens. We can name it additively by analogy with other inorganic dinuclear entities: di-μ-hydrido-bis(dihydridoboron). However, this compound is given much shorter, albeit less descriptive, preselected name diborane(6) [4, P-68.1.1.2.1].
The problem is, the composition of even simplest boranes does not tell us what their structure is. For example, the structure (b) is not the only possible diborane(4). A few years ago, its isomer (g) has been identified [5]:
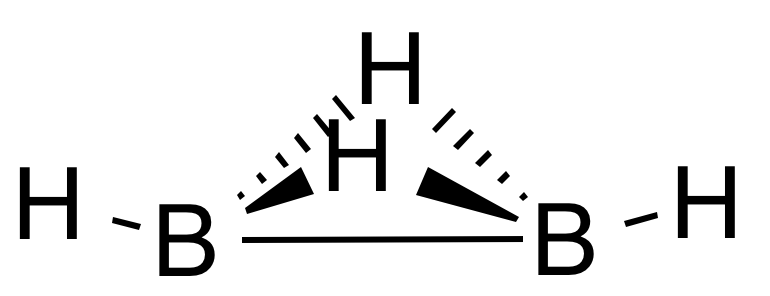 |
| (g) |
|---|
|
To differentiate between the two isomers, we can name (b) substitutively boranylborane and (g) additively di-μ-hydrido-dihydridodiboron(B—B) or di-μ-hydrido-bis(hydridoboron)(B—B). None of these names is short or elegant but they do describe the structure.
Polynuclear boranes tend to form cages where boron atoms occupy vertices of closed or open triangulated polyhedra, or deltahedra [3]. This means that any boron atom at a polyhedral vertex should be at least tetracoordinate. As you can imagine, simply indicating the composition won’t be nearly enough. λ-convention is not of much use here either as you’ll need to add “lambdas” to all boron atoms. To make the names of boranes more informative, they have to be modified with special structural descriptors [2, IR-6.2.3.2]:
| Descriptor | Parent hydride | Description of structure |
|---|---|---|
| closo | BnHn+2 | Closed deltahedral structure |
| nido | BnHn+4 | Nest-like non-closed polyhedral structure; a deltahedron with one vertex missing |
| arachno | BnHn+6 | Web-like non-closed polyhedral structure; a deltahedron with two vertices missing |
| hypho | BnHn+8 | Net-like non-closed polyhedral structure; a deltahedron with three vertices missing |
| klado | BnHn+10 | Open branch-like non-closed polyhedral structure; a deltahedron with four vertices missing |
To make a connection between a descriptor, the number of boron atoms and resulting deltahedron, it could be useful to have a look-up diagram, like the one given in the recent Recommendations [3, p. 358, Fig. 1]. One certainly needs such a diagram to work out the numbering of boron atoms in a cage.
According to the 2019 Recommendation 4 [3, p. 357],
It is recommended that structural descriptors closo, nido, and arachno, as defined in the 1990 recommendations, are retained. However, the polyhedral shapes for hypho and klado are structurally difficult to visualize, and the use of these descriptors is no longer acceptable.
So... they took two descriptors away without suggesting any substitute. I don’t know how it is helpful for (re)naming formerly hypho- and klado-boranes.
Let’s focus on the remaining three descriptors. For example, the closo structure (h) is an octahedral cage with all vertices occupied by boron atoms. Taking away one boron atom (and adding the appropriate number of hydrogens) brings about the nido structure (i); taking away one more yields the arachno structure (j).
 |
.png) |
.png) |
| (h) | (i) | (j) |
|---|
|
The name of the anionic boron hydride (h) is strikingly different from those of neutral boranes such as (i) and (j). Note it does not contain ‘(6)’; instead, the hydrogens in hydridoborates are indicated in a familiar additive way (that is, ligands first): hexahydrido-closo-hexaborate(2−). Maybe this is because the parentheses are needed now to accommodate the charge?
Boron atoms in polyboranes could be replaced by atoms of other elements, resulting in heteroboranes. They are named using skeletal replacement (aka ‘a’) nomenclature.
 |
.png) |
| (k) | (l) |
|---|
|
For example, replacing two boron atoms with carbons in closo-dodecaborane(12) (k) begets closo-1,2-dicarbadodecaborane(12) (l). Carbon-replaced boranes are commonly known as carboranes, another deprecation victim of the Recommendations [3].
Skeletal replacement in boranes is called subrogation [3, BN-4.2]. It’s a mystery how — and why — this atrocious legalese term and a host of its relatives (subrogate, subrogating, subrogated, unsubrogated) have found its way into IUPAC recommendations.
To sum up: borane nomenclature is an uneasy hybrid of pseudo-organic (parent hydride-based) and inorganic additive naming systems, with some specific features such as hydrogens in parentheses and closo/nido/arachno descriptors thrown in. It works and parts of it are “well entrenched in the <boron hydride related> literature” [3]; outside of the field, it’s bound to keep unhappy both inorganic and organic chemists.
| * | The systematic name ‘carbane’ is not recommended by IUPAC “because of the universal use of the name ‘methane’” [2, p. 85, Table IR-6.1]. |
| † | The IUPAC Recommendations [2, IR-6.2.3.1; 3] employ the terms stoichiometric names or compositional names for constructs à la ‘diborane(4)’, which is confusing given that elsewhere [2, IR-5.2] “stoichiometric names” refer to the likes of ‘diboron tetrahydride’. |
| ‡ | You may recall a similar situation with neopentane where a longest chain-based name ‘2,2-dimethylpropane’ is preferred to a perfectly symmetrical ‘tetramethylmethane’. |
References
- Leigh, G.J. (ed.) Nomenclature of Inorganic Chemistry, Recommendations 1990. Blackwell Science, 1990.
- Connelly, N.G., Hartshorn R.M., Damhus, T. and Hutton, A.T. Nomenclature of Inorganic Chemistry: IUPAC Recommendations 2005. Royal Society of Chemistry, Cambridge, 2005.
- Beckett, M.A., Brellochs, B., Chizhevsky, I.T., Damhus, T., Hellwich, K.-H., Kennedy, J.D., Laitinen, R., Powell, W.H., Rabinovich, D., Viñas, C. and Yerin, A. (2020) Nomenclature for boranes and related species (IUPAC Recommendations 2019). Pure and Applied Chemistry 92, 355—381.
- Favre, H.A. and Powell, W.H. Nomenclature of Organic Chemistry: IUPAC Recommendations 2013 and Preferred IUPAC Names. Royal Society of Chemistry, Cambridge, 2014.
- Chou, S.-L., Lo, J.-I., Peng, Y.-C., Lin, M.-Y., Lu, H.-C., Cheng, B.-M. and Ogilvie, J.F. (2015) Identification of diborane(4) with bridging B–H–B bonds. Chemical Science 6, 6872—6877.








No comments:
Post a Comment